Precision Medicine in Sarcoma Pinpoints Tropomyosin-Related Kinases
Peter J. Sciavolino, PhD, dives into the oncological roles of NTRK and TRK-targeted therapies

The tropomyosin-related kinases (Trk) are a family of tyrosine kinase receptors comprising at least three known family members: proteins TrkA, TrkB, and TrkC encoded by theNTRK1, NTRK2andNTRK3genes, respectively.1,2Like many other receptors, their tyrosine kinase activity links them with multiple intracellular signaling pathways, and although NTRKgenes are expressed in a number of different tissue types, their primary role appears to be in neuronal cell growth and differentiation and in mediating the effects of neurotrophins.1,2For example,NTRK2serves as a receptor for brain-derived neurotrophic factor (BNDF) and neurotrophin 4.2With the growing use of molecular profiling and targeted therapies in human cancers,NTRKgene rearrangements have more recently been recognized and implicated in several human cancers, including sarcomas.3,4The clinical development of targeted therapies for Trk also presents an opportunity for individualized treatment for tumors harboring molecular alterations.A new phase II global trial of entrectinib, a potent Trk inhibitor, will add additional clinical interest beyond typicalsarcoma clinical trialsin these genes.
Current Molecular Aspects of Sarcoma
Sant Chawla, MD, director of the Sarcoma Oncology Center and the Cancer Center of Southern California noted in an interview withTargeted Oncologythat while molecular testing is now extensively performed in sarcoma, the results, in terms of therapeutic implications, “are still not there.”
There are however, some exceptions, such as gastrointestinal stromal tumor (GIST) sarcoma, for which targeted therapies are available. In particular, the tyrosine kinase inhibitor (TKI) imatinib (Gleevec) is effective in about 70% to 80% of these tumors. There are specific molecular alterations in GIST sarcoma, such as chromosome 11 alterations, where imatinib is especially effective, with response rates of 90% or higher. Conversely, alterations in chromosomes 13 and 17 appear to be associated with very low response rates to imatinib, and new drugs are being sought for these more challenging sarcoma types. Chawla also cited the efficacy of sunitinib, another TKI, which is known to be more active in patients with chromosome 13 mutation GIST sarcoma. Overall, Chawla notes that, while there are about 75 different varieties of sarcomas, only a couple are presently treatable with targeted therapyone is GIST sarcoma (as mentioned) and a second type is myxoid liposarcoma, which has shown good responses to specific chemotherapies such as trabectedin, and approval for this indication is expected soon.
“Other types of liposarcomas express markers such as Mdm2, and we have new drugs which are inhibiting the Mdm2 pathwaysthese are antibodies as well as small molecules—and initial clinical trials are promising for inhibiting Mdm2 in those tumors,” Chawla said. Lastly, he cited therapeutic approaches aimed at other molecular markers, such as NY-ESO-1, which is highly expressed in synovial sarcoma, myxoid liposarcoma, and some other sarcoma types, as well as in ovarian cancers and melanoma. “We are taking this marker and creating an antibody, as well as an immunological approach directed against this antigen, NY-ESO-1.” Initial trials will include approaches using dendritic cells and tumor-infiltrating lymphocytes (TILs) directed against this molecular marker, which he sees as extremely promising.
Oncogenic Roles ofNTRK
Trk receptors may contribute to oncogenesis in sarcoma through molecular alterations that result in their inappropriate expression or activation. For example, BDNF and neurotrophin 4/5 expression has been found by immunostaining and quantitative real-time RT-PCR in uterine sarcoma cell lines and in primary tumor samples from patients with uterine leiomyosarcoma.5Interestingly, the use of a soluble ectodomain of TrkB, or a specific Trk inhibitor, K252a, significantly suppressed proliferation of these cell lines and led to increased-apoptosis; whereas, the addition of exogenous BDNF caused an increase in proliferation. The results suggested the importance of this paracrine Trk signaling pathway in uterine sarcoma as well as a potential benefit to Trk inhibition in sarcoma.5TrkB has also been previously shown to have the important pro-oncogenic effect of suppressing anoikis, or apoptosis resulting from a loss of cell-matrix interactions; thus its inappropriate expression in tumors may potentially contribute to an aggressive and metastatic behavior.6
Kinases that become inappropriately activated through gene rearrangements have also been recognized for some time as oncogenic drivers for both hematopoietic and solid tumor types, and their inhibition has been an attractive target for drug development.3Trk kinase fusions, in particular, have been described in multiple cancers including colorectal cancer, lung adenocarcinoma, salivary gland cancer, head and neck squamous cell cancer, glioblastoma multiforme, and thyroid cancer, and this has further fueled the development of pan-Trk inhibitor drugs for use in oncology.3,4
In accordance with the potential for Trk fusions to be used as molecular targets in cancer, Trk inhibition has been shownin vitroto inhibit the proliferation of cell lines expressing Trk fusions. In a human colorectal cancer cell line (KM-12) driven by constitutively active TrkA fusion TPM3-NTRK1, entrectinib (a pan-Trk/ROS1/ALK inhibitor also known as RXDX-101) exhibitedin vitroantiproliferative activity with an IC50 of 17 nM, accompanied by inhibition of TrkA phosphorylation and concomitant inactivation of downstream effectors, PLCg1, AKT, and ERK, as well as cell cycle arrest and apoptosis. In mice bearing KM-12 xenografts, treatment with entrectinib resulted in tumor regression and durable stasis under either intermittent or continuous dosing regimens, accompanied by sustained intratumoral inhibition of phospho-TrkA and PLCg1.7In a second preclinical study,in vivoeffects of entrectinib as a single agent and in combination with the chemotherapeutic agents irinotecan and temozolomide (Irino-TMZ) was tested in TrkB-expressing neuroblastoma (NB) xenografts. Significant tumor growth inhibition was observed compared with controls when entrectinib was used in combination with Irino-TMZ and when used as single agent therapy.8
A recent clinical study details strong clinical response to a Trk inhibitor by an advanced sarcomapatient. An oncogenic Trk fusion was found in a patient with soft tissue sarcoma, consisting of exons 1-2 of the lamin A/C gene, and exons 11-17 of theNTRK1gene (LMNA-NTRK1).9Further, an examination of the patient’s tumor sample using a proximity ligation assay approach to detect functional signaling complexes demonstrated a robust signaling activity for the LMNA-TrkA fusion protein, but only weak signaling for the normal TrkA protein, as expressed in blood vessel. The findings thus suggested that the LMNA-TrkA fusion protein acted as a key oncogenic driver for this patient’s tumor, and thus the patient could be rationally treated with a pan-Trk inhibitor drug (LOXO-101); as such, the patient was ultimately enrolled in a phase I trial of this agent (100 mg twice daily).7The results showed that the patient exhibited improvement in the tumor-related symptom of exertional dyspnea and reduction in CA125 marker levels by Cycle 1 of treatment, with no observed treatment-related adverse events (AEs). By Cycle 2, computed tomography (CT) scan showed a partial response, with marked improvement in multiple pulmonary metastases as assessed by RECIST 1.1 criteria, and by Cycle 5 (after 4 months of dosing), there was almost complete regression of the largest tumor. Collectively, although further follow-up is needed to assess the durability of patient’s response, the results showed that Trk fusions could constitute potentially actionable molecular targets for the subset of soft tissue sarcoma patients harboringNTRKrearrangements.9
Molecular Analysis ofNTRKin Sarcoma: Growing Interest
Herbert H. F. Loong, MBBS, MRCP, FHKCP, FHKAM, is a specialist in medical oncology and assistant clinical professor in the Department of Clinical Oncology at Prince of Wales Hospital in The Chinese University of Hong Kong. In an interview withTargeted Oncology, Loong noted that no large published series are currently exploring overall frequency ofNTRKgene rearrangements in sarcoma.
“Previously, TrkC fusions were known for a rare sarcoma known as infantile congenital fibrosarcoma. However, with the emergence of large-scale screening through next-generation sequencing (NGS) and other platforms, recent data from FoundationOne have detectedNTRK1andNTRK3fusions in 8 of their 1272 soft-tissue sarcoma samples.8This is believed to be an underestimate, and within the community, we believe thatNTRKfusions occur more frequently,” Loong said. However, “It is important to note that sarcomas are extremely heterogenous in their own right, and as yet, there have not been conclusive studies that have identified which histological sarcoma subtype (aside from congenital fibrosarcoma) has a higher incidence.”
Loong believes that molecular testing for sarcoma is becoming more common but is now mainly used as a confirmatory test in addition to traditional hematoxylin-eosin (H&E) and immunohistochemical (IHC) methods. “Given the heterogeneity of sarcomas with over 90+ subtypes in the WHO guidelines, and the relative low incidence of sarcomas in general, molecular testing in this disease is taking a much more prominent role. In particular, about 10% to 15% of all sarcomas bear a recurrent chromosomal translocation, and recent technological advances have led to changes in diagnosis and classification of these tumors,” he said.
In a study of 203 adolescent and young adult sarcomas, three NTRK1 fusions were found, suggesting a prevalence of 1.5%.10These patients had multipleNTRK1fusion partners, and were found in an angiosarcoma, a leimyosarcoma, and an undifferentiated sarcoma. However, given the large size of theNTRKgenes, particularly large intronic regions ofNTRK2andNTRK3, as well as the many fusion partners, it is likely that there may be higher incidence of fusions that are missed using currently available testing methodologies. Recent diagnostic technology improvements use hypothesis-free RNA-based testing to eliminate both false-negative issues associated with large intronic repetitive regions and a priori assumptions about gene partner or fusion location.11This technology has been developed for use in Ignyta’s phase IINTRK/ROS1/ALKbasket trial across solid tumors including sarcomas, of the compound entrectinib.
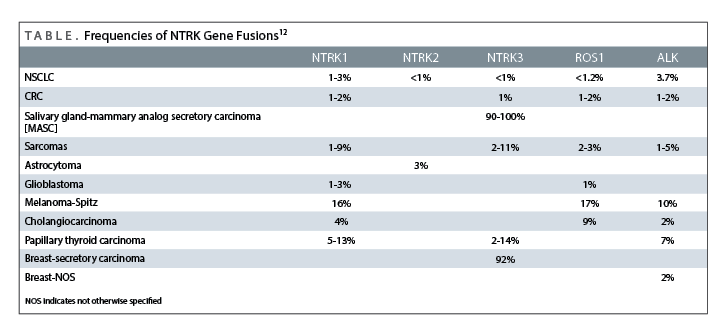
TRK-Targeted Therapies Offer New Treatment Possibilities
In a late-breaking abstract at this year’s European Society for Medical Oncology (ESMO) meeting, phase I results with entrectinib in patients with solid tumors having molecular rearrangements in theNTRK 1/2/3, ROS1,orALKgenes were presented.12The safety, pharmacokinetics and recommended phase II dosing (RP2D) for entrectinib was investigated across two phase I clinical trials, using a 3+3 dose escalation, with entrectinib given intermittently or with once daily (QD) dosing at ranges between 100 to 1600 mg/m2and at fixed doses of 600 mg and 800 mg. RECIST v1.1 criteria were used to evaluate antitumor activity of entrectinib in the study. Overall, entrectinib was well tolerated. The most frequent (>10% incidence) treatment-related AEs were fatigue/asthenia, dysgeusia, paraesthesia, nausea, and myalgia. In addition, three treatment-related serious AEs were reported, two of which occurred above the RP2D, with the remainder, a case of Grade 2 fatigue and fall, occurring at 600 mg fixed.12
Notably, an Overall Response Rate of 72% (13/18 patients) and a disease control rate of 89% (16/18 patients) was observed in the population withNTRK,ROS1andALKgene rearrangements. Of special interest was a nonsmall cell lung cancer patient withNTRK1rearrangement who had a partial response along with complete resolution of numerous CNS metastatic lesions.10The favorable results with entrectinib seen in the two trials, with good tolerability, predictable pharmacokinetics, and antitumor activity in a population of patients with relevant molecular alterations (includingNTRKalterations) have prompted the recent initiation of a global phase II basket study of entrectinib (STARTRK-2 trial).
“A basket trial is a new and evolving form of clinical trial design where the predicted hypothesis is that the presence of a particular molecular marker predicts response to a targeted therapy independent of tumor histology,” Loong explained. “Essentially, patients of all histologies (although the design can be to incorporate cancers, which are known to have more of a particular mutation/aberrations instead of all-comers) have their tumors screened for particular molecular aberrations, and patients who have that particularly actionable mutation/aberration are permitted participation. The success of a basket trial depends in large part on the strength of the data linking the target and targeted therapy. For this trial design to work, two key conditions must be met: the tumor must depend on the target pathway, and the targeted therapy must reliably inhibit the target.”
Loong also elaborated on the benefit of such a trial for identifying rare actionable mutations, in smaller groups of patients. “In the era of molecular profiling, this type of approach has significant advantages for rare tumors and also for tumors with rare molecular alterations. Many a time, it is through these large studies that early signals of efficacy are seen, and if that is the case, the trial can have an expansion cohort of this particular histological type. It is extremely difficult to set up and recruit patients in stand-alone trials of particular histological subtypes in rare tumors, due to the lack of patients that can be accrued and the costs/efficacy ratio involved.”
Chawla noted that his own group will also be involved with the entrectinib basket study, which will involve molecular testing forNTRKgene rearrangements in samples from patients with sarcoma at his institution. He noted the positive data that have been seen with entrectinib in patients withNTRKrearrangements in colon cancer, lung cancer, and salivary gland cancer, but which has not yet been tested in sarcomas. He expects that (as has been the case with other targeted treatments in basket trials) any sarcoma patients identified withNTRKgene rearrangements will be eligible for treatment, in line with what he calls Ignyta’s ‘Dx-Rx’ strategywith mutational diagnosis first, followed by rational targeted treatments.
For more information on the phase II trial for entrectinib in patients with NTRK,ROS1, andALKgene rearrangements, please visitwww.STARTRKtrials.com.
References
- Vaishnavi A, Le AT, Doebele RC. TRKing down an old oncogene in a new era of targeted therapy.Cancer Discov.2015;5(1):25-34.
- Gupta VK, You Y, Gupta VB, Klistorner A, Graham SL. TrkB receptor signalling: implications in neurodegenerative, psychiatric and proliferative disorders.Int J Mol Sci.2013;14(5):10122-10142.
- Stransky N, Cerami E, Schalm S, Kim JL, Lengauer C. The landscape of kinase fusions in cancer.Nat Commun.2014;5:4846.
- Narayanan R, Yepuru M, Coss CC, et al. Discovery and preclinical characterization of novel small molecule TRK and ROS1 tyrosine kinase inhibitors for the treatment of cancer and inflammation.PLoS One.2013;8(12):e83380.
- Makino K, Kawamura K, Sato W, Kawamura N, Fujimoto T, Terada Y. Inhibition of uterine sarcoma cell growth through suppression of endogenous tyrosine kinase B signaling.PLoS One.2012;7(7):e41049.
- Douma S, Van Laar T, Zevenhoven J, Meuwissen R, Van Garderen E, Peeper DS. Suppression of anoikis and induction of metastasis by the neurotrophic receptor TrkB.Nature.2004;430(7003):1034-1039.
- Radhika I, Golden RK, Naraparaju K, et al. The Trk inhibitor RXDX-101 enhances the efficacy of temozolimide and irinotecan in a xenograft model of neuroblastoma. Abstract 5390. American Association of Cancer Research (AACR) Meeting; April 15, 2015: Philadelphia, Pennsylvania.
- Anderson D, Ciomei M, Banfi P, et al. Inhibition of Trk-driven tumors by the pan-Trk inhibitor RXDX-101. Abstract 3010. EORTC-NCI-AACR “Molecular Targets and Cancer Therapeutics” Conference, Nov 18, 2014: Barcelona, Spain.
- Doebele RC, Davis LE, Vaishnavi A, et al. An Oncogenic NTRK fusion in a patient with soft-tissue sarcoma with response to the tropomyosin-related kinase inhibitor LOXO-101.Cancer Discov.doi:10.1158/2159-8290.CD-15-0443.
- Morosini D, Chmielecki J, Goldberg M, et al. Comprehensive genomic profiling of sarcomas from 267 adolescents and young adults to reveal a spectrum of targetable genomic alterations. Abstract 11020. American Society of Clinical Onology (ASCO) Meeting; June 4, 2015: Chicago, Illinois.
- Zheng Z, Liebers M, Zhelyazkova B, et al. Anchored multiplex PCR for targeted next-generation sequencing.Nature Medicine2014; 20:14791484.
- S. Siena. Entrectinib (RXDX-101), an oral pan-Trk, ROS1, and ALK inhibitor in patients with advanced solid tumors harboring gene rearrangements. Late Breaking Abstract (LBA 29). European Society for Medical Oncology (ESMO) Meeting; September 27, 2015: Vienna, Italy.
Glofitamab Plus Chemo Improves Survival vs Rituximab in R/R DLBCL
April 16th 2024The phase 3 STARGLO trial met its primary end point, improving overall survival in patients with relapsed/refractory diffuse large B-cell lymphoma with glofitamab and chemotherapy vs rituximab and chemotherapy.
Read More
Biomarker Testing Paves the Way for Better Targeted Therapies in NSCLC
April 16th 2024At a live virtual event, Edward S. Kim, MD, MBA, discussed the evolving landscape of biomarker testing before making treatment decisions for patients with early-stage non–small cell lung cancer (NSCLC).
Read More
FDA Accepts IND for UGN-103 in Low-Grade Intermediate-Risk NMIBC
April 15th 2024An investigational new drug application for UGN-103 was accepted by the FDA. A phase 3 study to assess the safety and efficacy of the agent in low-grade intermediate-risk non-muscle invasive bladder cancer is anticipated.
Read More
Glofitamab Plus Chemo Improves Survival vs Rituximab in R/R DLBCL
April 16th 2024The phase 3 STARGLO trial met its primary end point, improving overall survival in patients with relapsed/refractory diffuse large B-cell lymphoma with glofitamab and chemotherapy vs rituximab and chemotherapy.
Read More
Biomarker Testing Paves the Way for Better Targeted Therapies in NSCLC
April 16th 2024At a live virtual event, Edward S. Kim, MD, MBA, discussed the evolving landscape of biomarker testing before making treatment decisions for patients with early-stage non–small cell lung cancer (NSCLC).
Read More
FDA Accepts IND for UGN-103 in Low-Grade Intermediate-Risk NMIBC
April 15th 2024An investigational new drug application for UGN-103 was accepted by the FDA. A phase 3 study to assess the safety and efficacy of the agent in low-grade intermediate-risk non-muscle invasive bladder cancer is anticipated.
Read More
2 Commerce Drive
Cranbury, NJ 08512
All rights reserved.