Evolving Paradigms In Bladder Cancer: Emerging Therapies
This is the "Emerging Therapies" section of the current issue of Evolving Paradigms In Bladder Cancer.
TABLE 3
).
Evolving Paradigms In Bladder Cancer
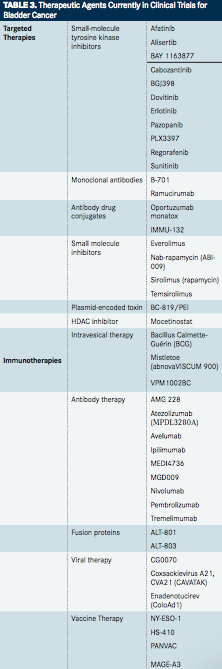
Targeted Therapies
Tyrosine Kinase Inhibitors
Sunitinib is a tyrosine kinase inhibitor that blocks the vascular epidermal growth factor receptor (VEGFR), which plays an important role in angiogenesis and tumor growth. A report found that sunitinib treatment in patients with metastatic bladder cancer results in a clinical response after recurrence.54Investigators at the University of Michigan are studying the effect of sunitinib treatment following BCG delivery in patients with high-risk nonmuscle-invasive bladder cancer (Sutent trial). While about half of patients with nonmuscle-invasive disease experience a complete response to BCG therapy alone at 3 months, up to one-third of these patients develop recurrent or progressive disease. The Sutent trial will evaluate complete response at 3 months and 6 months, as well as recurrence at 3 years, following combination treatment with BCG and sunitinib.55
Cabozantinib is another receptor tyrosine kinase inhibitor that targets VEGFR and slows angiogenesis. Previous studies have demonstrated its use in the treatment of medullary thyroid cancer, prostate cancer, and ovarian cancer. A phase II study being conducted at the National Cancer Institute (NCI) is investigating its use in advanced and/or metastatic urothelial carcinomas. Overall response rate is the primary outcome of the study.56In addition to single-agent cabozantinib, a recently initiated phase I trial will also evaluate combination regimens of cabozantinib with immune checkpoint inhibitor therapy in patients with advanced or metastatic bladder cancer or other genitourinary tumors.57Patients will receive either single-agent cabozantinib, or the agent in combination with the PD-1 antibody nivolumab alone, or the agent in combination with both nivolumab and ipilimumab. Primary endpoint is the dose-limiting toxicity for each of these combinations.57
Pazopanib is a tyrosine kinase inhibitor that blocks VEGF and is currently being studied in kidney cancer. Combination treatment with pazopanib and paclitaxel was shown to be favorable in a phase I trial. Therefore, an ongoing phase II trial is evaluating pazopanib and weekly paclitaxel in patients with first recurrence following chemotherapy and with TCC of the urothelium, with objective response rate according to response evaluation criteria in solid tumors (RECIST) as the main outcome.58Outcomes from this trial have been reported for the first 28 evaluable patients and indicate activity of the combination regimen, with an overall response rate of 50%, including 11% complete responses. The median progression-free survival (PFS) was 6 months, and median OS was 8 months.59
Alisertib is a selective, small molecule inhibitor of aurora kinase A, a protein involved in mitosis and cell proliferation. Overexpression of aurora kinase A has recently been reported in bladder cancer, and dysregulation of spindle checkpoints is common in urothelial carcinoma. The Fondazione IRCCS Istituto Nazionale dei Tumori, Milano will conduct a randomized, phase II trial to study the effects of alisertib plus or minus paclitaxel versus placebo plus paclitaxel on the 2-month response rate and PFS of patients with relapsed or refractory TCC of the bladder and urothelial tract.60
Erlotinib is a reversible tyrosine kinase inhibitor that acts upon epidermal growth factor receptor (EGFR), which is commonly mutated and overexpressed in several types of cancer. EGFR activates the PI3K-AKT-mTOR and Ras-MAPK pathways, which regulate cell growth. Erlotinib is most well-known for its use in the treatment of nonsmall cell lung cancer (NSCLC) and pancreatic cancer. A multicenter, randomized, phase II trial will begin recruiting in October 2014 to investigate the effect of pretreatment with erlotinib versus placebo before surgery (TURBT) on tumor cell growth in patients with bladder cancer. Patients with stages T1-T3 bladder cancer, as well as patients with recurrent bladder cancer, will be eligible for the study. The main outcome will be phosphorylation of EGFR in the bladder tissue adjacent (normal appearing) to the tumor.61
Afatinib is a second-generation tyrosine kinase inhibitor that irreversibly inhibits human epidermal growth factor receptor 2 (HER2) and EGFR, both of which play crucial roles in cancer cell growth and survival. Similar to EGFR, HER2 is a protein that is commonly overexpressed in cancer and is associated with more aggressive disease. The University of Chicago is conducting a trial to study the effect of afatinib treatment on PFS in patients with refractory urothelial cancer. Patients enrolled in this study have cancers that are unable to be removed surgically and that have progressed following treatment with standard first-line chemotherapy. Similar to the erlotinib study, investigators expect that treatment with afatinib may retard tumor cell growth and/or promote cancer cell death.62
Dovitinib is a receptor tyrosine kinase inhibitor that binds strongly to FGFR3, which plays a role in cell proliferation. FGFR3 is commonly mutated in bladder cancer, leading to its constitutive activation and resulting in aberrant cell proliferation. A multicenter, phase II trial is assessing the use of oral dovitinib in BCG-refractory urothelial carcinoma patients harboring tumors with FGFR3 mutations or overexpression and who are ineligible for or refusing cystectomy. The primary outcome of the study is the proportion of patients with a complete response 6 months following treatment with dovitinib.63
Monoclonal Antibodies
Ramucirumab is a monoclonal antibody with high specificity for the extracellular domain of the vascular endothelial growth factor receptor tyrosine kinase VEGFR-2 that mediates broad inhibition of VEGFR-2 and thus angiogenesis. Data from a randomized phase II study have shown that adding ramucirumab to docetaxel significantly increased PFS of patients with advanced urothelial carcinoma with progression after platinum therapy (22 weeks vs 10.4 weeks on docetaxel alone).64The addition of ramucirumab to docetaxel is currently being investigated in a large, randomized phase III trial (RANGE) in patients with urothelial cancer who failed previous therapy.65Patients with advanced bladder cancer are also eligible for a phase I trial that is evaluating the combination of ramucirumab with the immunotherapeutic agent pembrolizumab, which targets PD-1.66
Small Molecule Inhibitors
Everolimus is an mTOR inhibitor that has been used in the treatment of several cancers, including breast, pancreatic, and kidney. mTOR is a serine/threonine protein kinase in the PI3K pathway that is involved in cell growth, proliferation, motility, and survival. It also plays a role in protein synthesis and transcription. Patients with tumors containing activating mutations in mTOR maybe particularly responsive to treatment with mTOR inhibitors, as suggested by the case report of a patient with advanced bladder cancer who experienced an exceptional response of 14 months duration to a combination of everolimus and pazopanib. Whole exome sequencing revealed the simultaneous presence of two activating mTOR mutations in the urothelial carcinoma of this patient.67Three fully accrued trials are currently evaluating everolimus in bladder cancer. These include a multicenter, randomized, phase II trial investigating everolimus alone or everolimus plus paclitaxel as first-line therapy in cisplatin-ineligible patients with advanced urothelial carcinoma.
The primary outcome is response rate (complete response, partial response, or stable disease) at 4 months from initiation of treatment.68The second study is a phase I trial investigating the safety of standard chemotherapy (gemcitabine and cisplatin) plus everolimus at different dose levels in patients with advanced urothelial cancer. The addition of everolimus to cisplatin has previously been shown to enhance the killing of cancer cells, thus suggesting its therapeutic potential in cancer treatment. The primary outcome of this study is doselimiting toxicity for everolimus in combination treatment with gemcitabine and cisplatin.69The third study is a phase I/II study at Memorial Sloan Kettering Cancer Center investigating different doses of oral everolimus paired with intravesical delivery of gemcitabine in patients with primary or secondary carcinoma in situ of the bladder and who are refractory to BCG. Dose-limiting toxicity and the maximum tolerated dose will be assessed for this combination therapy as part of the phase I portion of the trial. The primary outcome of the phase II portion of the trial is the proportion of patients who experience complete response at 1 year following initiation of therapy.70
The mTOR inhibitor temsirolimus showed clinical efficacy in the French, multicenter, phase II VESTOR study in patients with relapsed bladder cancer.71 Among 36 evaluable patients, 50% experienced nonprogression at 2 months, defined as the sum of partial response, complete response, and stable disease.72 Additional analyses are ongoing to identify mutations that may predict sensitivity to temsirolimus.72
Nanoparticle albumin-bound rapamycin (Nab-rapamycin; ABI009) is an mTOR inhibitor that is currently being studied for its use in the treatment of nonmuscle-invasive bladder cancer. Patients included in the study have either recurrent nonmuscle-invasive TCC of the bladder or are refractory to BCG treatment. This study is a phase I/II trial. Phase I primary outcomes are safety and tolerability, while the phase II outcome is complete response rate.73
Immunotherapies
Intravesical Therapies
Checkpoint Inhibitor Therapies
BCG therapy is the most commonly used immunotherapy in bladder cancer treatment. BCG is a live, attenuated strain of Mycobacterium bovis and is the only therapy approved by the FDA for carcinoma in situ of the bladder. Cystectomy is often accompanied by BCG intravesical therapy in patients with bladder cancer because it has been shown to reduce the risk of recurrence and tumor progression.32Several agents mentioned in this section are supplements of BCG therapy.
Checkpoint inhibitors are monoclonal antibodies directed against inhibitory immune receptors or their ligands. These so-called checkpoints control T-cell activation and normally maintain selftolerance and prevent autoimmunity; however, their upregulation in cancers can prevent an anticancer immune response. The use of checkpoint inhibitors can therefore augment the ability of the patient’s immune system to eliminate tumor cells, resulting in long-lasting responses. Key checkpoints that are pursued therapeutically include the PD-1 and cytotoxic T-lymphocyte-associated antigen-4 (CTLA-4) pathways.74
Ipilimumab is an antiCTLA-4 antibody that has shown promise as a neoadjuvant therapy for patients with clinically localized bladder cancer undergoing radical cystectomy. Data have become available from a multicenter phase II trial evaluating the combination of chemotherapy (gemcitabine, cisplatin) with ipilimumab in the treatment of metastatic urothelial carcinoma.75The overall response rate among 36 treated patients was 23%, including five complete responses, and median PFS was 8 months.76
Atezolizumab is an antibody against PD-L1, a ligand for the PD-1 pathway. When the body encounters a foreign antigen, there is a proliferative response of antigen-specific CD8+ T cells in the lymph nodes and spleen. The PD-1 pathway reduces proliferation of these T cells upon binding of PD-L1 to the PD-1 receptor. Neutralizing PD-L1 restores the accumulation of CD8+ T cells that can counteract tumor cells. Phase I and II study results with atezolizumab in patients with advanced or metastatic bladder cancer have demonstrated tumor shrinkage in 43% of patients42 and an OS of 11.9 months in patients PD-L1expressing tumors, respectively.44The phase II study also reported high response rates to the agent among heavily pretreated patients with metastatic urothelial carcinoma, who had progression on platinum-based chemotherapy; overall responses were 15% in the entire cohort, and 18% and 27%, respectively, in patients with ≥1% and ≥5% tumor PD-L1 expression.77
Two phase III trials of atezolizumab are currently recruiting patients with bladder cancer,78,79and an open-label, single-arm, phase IV expanded access study has been initiated to provide atezolizumab to patients with locally advanced or metastatic platinum-resistant urothelial carcinoma.80The phase III IMvigor211 trial will compare the efficacy of atezolizumab with chemotherapy in patients with platinum-resistant advanced or metastatic bladder cancer, with OS as the primary outcome measure.78The second phase III trial, IMvigor010, is investigating efficacy and safety of adjuvant atezolizumab compared with observation in patients with PD-L1positive muscle-invasive bladder cancer who are at high risk for recurrence after cystectomy. The primary efficacy endpoint of this trial is disease-free survival.79
Nivolumab is an antibody against PD-1, which is currently being studied by Bristol-Myers Squibb in a phase II trial in patients with metastatic or unresectable bladder cancer, with objective response rate per RECIST as the primary endpoint. This trial has completed accrual.81A second phase I/II clinical trial is evaluating nivolumab in several cancers, including advanced or metastatic triple-negative breast cancer (TNBC), gastric cancer, pancreatic adenocarcinoma, small cell lung cancer, and bladder cancer. This trial compares treatment with nivolumab alone versus nivolumab plus ipilimumab and evaluates the objective response rate up to 17 months following treatment.82
The antiPD-1 antibody pembrolizumab showed promising results in patients with advanced or metastatic urothelial carcinoma who participated in the phase I KEYNOTE-12 study in patients with advanced solid tumors, with updated results reporting an overall response rate of 25% in 28 evaluable patients, and of 38% in patients with PD-L1–positive tumors; the 12-month PFS rate was 19%.83
The phase III KEYNOTE-45 study comparing pembrolizumab with chemotherapy in patients with advanced urothelial cancer has completed accrual; primary outcomes are OS and PFS, with follow-up of up to 27 months.84The ongoing phase II KEYNOTE-52 trial is evaluating overall response rate to pembrolizumab in patients with advanced or metastatic urothelial cancer ineligible for cisplatin therapy.85A second phase II trial will assess pembrolizumab as maintenance therapy after initial chemotherapy,86and a third phase II monotherapy study (KEYNOTE-057) will investigate the agent in patients with high-risk nonmuscle invasive bladder cancer.87Patients with bladder cancer are also eligible for the expansion phase of a phase I/II study of pembrolizumab in combination with the small molecule kinase inhibitor PLX3397.88
Phase I study data have also become available for the antiPDL1 checkpoint agent avelumab, with a reported overall response rate of 15.9% in patients with refractory metastatic urothelial carcinoma, including one complete response.89,90The disease control rate was 59.1%. Of patients with PD-L1expressing tumors, 70% were progression-free at 12 weeks, compared with 45.5% of patients with PD-L1–negative cancers.89,90The phase III JAVELIN Bladder 100 study will compare avelumab to best supportive care in patients with locally advanced or metastatic urothelial cancer as maintenance treatment following first-line chemotherapy.91
Fusion Proteins
ALT-801 is a recombinant fusion protein of interleukin-2 (IL-2) and soluble T-cell receptor directed against an antigen driven by the tumor suppressor p53. Tumor cells presenting p53/major histocompatibility complexes (MHC) are bound by ALT-801, resulting in the IL-2 moiety of the fusion protein stimulating natural killer (NK) cells and T-cell cytotoxic immune responses against the tumor cells. Data from a phase Ib/II trial evaluating the addition of ALT-801 to either gemcitabine alone or to the combination of gemcitabine and cisplatin in patients with muscle-invasive or metastatic urothelial cancers have shown clinical activity of the agent.92,93 The overall response rate was 35%, and complete responses were seen in both treatment groups. A second phase I/II study is studying the addition of ALT-801 to gemcitabine in patients with nonmuscle-invasive bladder cancer who have failed BCG therapy.94
ALT-803 is a fusion protein complex of superagonist interleukin-15 (IL-15) and a soluble, dimeric IL-15 receptor alpha Fc fusion protein. ALT-803 binds to the IL-2/IL-15 receptor beta-common gamma chain receptor on NK and CD8+ T cells, activating these immune cells and inducing their expansion. A phase Ib/II, multicenter study is determining the effect of combination therapy with BCG and ALT-803 in patients with nonmuscle-invasive bladder cancer who have not received prior BCG therapy. Safety, maximum tolerated dose, and recommended dose are the primary outcomes.95
Viral Therapies
CG0070 is an oncolytic adenovirus that encodes granulocyte macrophage-colony stimulating factor (GM-CSF). This virus selectively infects tumor cells and replicates in them, potentially leading to tumor cell lysis. At the same time, the GM-CSF expressed by the virus may stimulate cytotoxic T-cell responses against tumor cells. A dose-escalation study of intravesical CG0070 in patients with superficial bladder cancer who had failed BCG therapy demonstrated a 64% and 23% complete response rate among patients receiving multiple and a single dose.96
Vaccine Therapies
Outcomes from the ongoing fully accrued phase II/III efficacy trial of CG0070 in nonmuscle-invasive bladder cancer (BOND) are expected in 2018.97Patients included in the study have TCC, bladder cancer, carcinoma in situ, or carcinoma in situ with papillary tumors. The phase II outcome of this study is the proportion of patients achieving complete response 9 months after treatment. The phase III outcome is the proportion of patients with durable complete response 15 months following treatment.97A second phase III trial of CG0070 (BOND2) is evaluating the agent in patients with high-grade nonmuscle invasive bladder cancer who failed BCG therapy and refused cystectomy.98
NY-ESO-1 is a cancer-testis antigen found in normal testis and overexpressed in many tumor types. An NY-ESO-1 peptide vaccine has been developed to stimulate the immune system to mount antitumor responses to cells expressing NY-ESO-1 antigen, resulting in tumor cell lysis. A phase I trial is being conducted to evaluate the safety and dosing schedule for NY-ESO-1 vaccine therapy with or without sirolimus treatment in patients with solid tumors expressing NY-ESO-1, including recurrent and stage IV bladder cancers. Secondary outcomes will measure NYESO-1specific cellular and humoral immunity.99
HS-410 is an allogenic urothelial bladder cancer cell vaccine that expresses a recombinant, secretory heat shock protein gp96 fused with an Fc domain from an immunoglobulin (Ig). HS-410 administration results in continuous secretion of gp96-Ig in irradiated tumor cells, which enhances presentation of tumor-associated antigens to cytotoxic T cells and induces an antitumor response. Results from the phase I portion from a phase I/II trial investigating administration of HS-410 in patients with nonmuscle-invasive bladder cancer have become available, demonstrating safety and tolerability of the agent, and suggesting clinical activity, with 7/10 patients remaining recurrence free after 1 year.100,101The ongoing phase II portion of the study is fully enrolled and will evaluate if disease-free survival is improved 1 year after treatment with BCG combined with HS-410 compared with placebo.100
PANVAC is a viral cancer vaccine that expresses mucin-1 (MUC1) and TRIad of COstimulatory Molecules (TRICOMTM), proteinsoverexpressed by many cancers. When cancer cells become infected with PANVAC, MUC-1, and TRICOM are expressed and presented along with other tumor-associated antigens. The immune system associates these proteins with viral proteins and mounts a response to them, potentially killing tumor cells. A randomized, phase II study is investigating whether or not the addition of PANVAC to BCG therapy has improved disease-free survival over the course of 5 years compared with BCG therapy alone. Patients included in the study have high-grade nonmuscle-invasive bladder cancer and have failed prior BCG therapy.102
MAGE-A3 is a peptide cancer vaccine comprised of a peptide from the human melanoma antigen A3, which is expressed in a variety of cancer types, including bladder cancer. MAGE-A3 acts as an immunostimulant by inducing a cytotoxic T-cell response against tumor cells that express MAGE-A3. A randomized, placebo-controlled, phase II trial is evaluating the safety and efficacy of MAGE-A3 plus the vaccine adjuvant AS-15 in patients with MAGE-A3+ muscle-invasive bladder cancer after cystectomy. The primary outcome of the study is disease-free survival 5 years following cystectomy.103

Advances in Subsequent Therapies Shake Up Sequencing of ccRCC Treatment
April 25th 2024With the approval of belzutifan and other newer data for treating patients with recurrent renal cell carcinoma, the state of subsequent therapies is advancing beyond the reuse of frontline options with impacts on duration of response and quality of life.
Read More
Novel Approaches Focus on Limiting Toxicity in Older Patients With ALL
April 22nd 2024The major challenges for clinicians treating older patients with acute lymphoblastic leukemia surround the emergence of resistance to existing therapies and the toxicities associated with current chemotherapies.
Read More
Ornstein Advises on Starting Dose and Management of Lenvatinib in RCC
April 21st 2024During a Case-Based Roundtable® event, Moshe Ornstein, MD, MA, provided guidance on dosing and toxicity concerns in a patient treated with lenvatinib plus pembrolizumab for advanced renal cell carcinoma.
Read More