Current Approaches to Diagnosis and Risk Stratification in CLL
Recent years have seen major advances in diagnostic approaches in CLL, and disease assessment may now include analysis of multiple genetic mutations in addition to recurrent cytogenetic changes. Furthermore, with the development of novel treatment approaches, the significance of prognostic markers is shifting.
1A CLL diagnosis is established by the presence of more than 5x109/L peripheral lymphocytes co-expressing CD5, CD19, and CD23, and weakly expressing CD20, CD79b, and surface immunoglobulin.2Small lymphocytic lymphoma (SLL), in which the same leukemic cell population is mostly restricted to the bone marrow and lymphoid tissues, represents a clinical variant of CLL and is similarly managed.3
CLL is the most common adult leukemia in Western countries. Its incidence increases with age, and with aging populations, its prevalence and mortality in Western countries will continue to rise. Improved diagnostic methods and more frequent blood testing have also led to increasing identification of early-stage CLL among younger patients.2,4Approximately 10% to 15% of patients with CLL are younger than 55 years.2,4
Recent years have seen major advances in diagnostic approaches in CLL, and disease assessment may now include analysis of multiple genetic mutations in addition to recurrent cytogenetic changes.1Furthermore, with the development of novel treatment approaches, the significance of prognostic markers is shifting. Awareness of current approaches to CLL diagnosis and assessment of disease criteria relevant to current risk stratification and treatment selection strategies is therefore prerequisite to tailor treatment for each patient.
EPIDEMIOLOGY AND RISK FACTORS
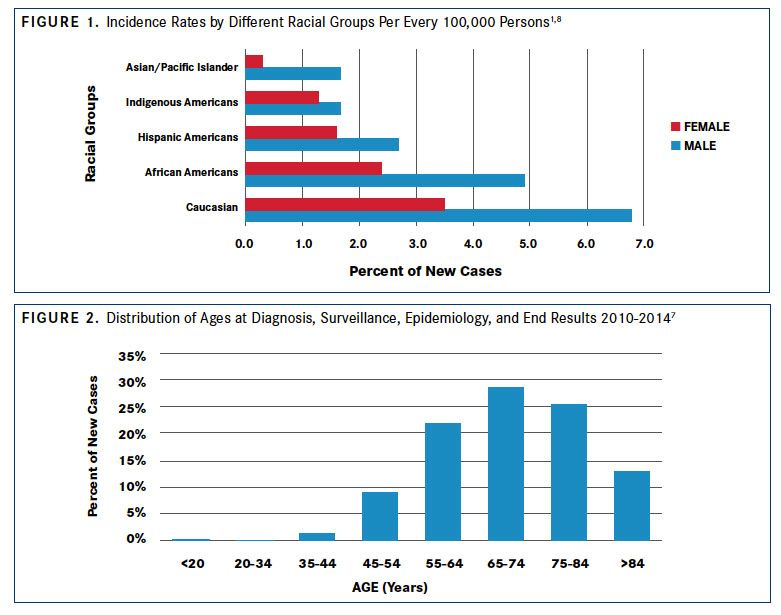
The incidence of CLL varies between geographic locations and is lower in Eastern Asian populations, such as China, Korea, and Japan, than in Western countries.1Lower incidence of CLL is maintained in Asian individuals emigrating to Western countries and their progeny.5With an age-adjusted incidence of approximately 4 per 100,000 inhabitants in Europe and the United States, CLL is the most common adult leukemia in adults in Western countries.2In the United States, CLL accounts for approximately one-third of all annual new leukemia diagnoses (20,110 of 62,130 in 2017).6,7According to estimates based on the US National Cancer Institute Surveillance, Epidemiology, and End Results (SEER) database, CLL is most frequent in white populations in the United States (6.8 per 100,000 men and 3.5 per 100,000 women), followed by African Americans (4.9 per 100,000 men and 2.4 per 100,000 women).1,8Rates are lower among Hispanic Americans (2.7 per 100,000 men and 1.6 per 100,000 women), Indigenous Americans (1.7 per 100,000 men and 1.3 per 100,000 women), and individuals of Asian or Pacific Island descent (1.7 per 100,000 men and 0.3 per 100,000 women) (FIGURE 11,8).
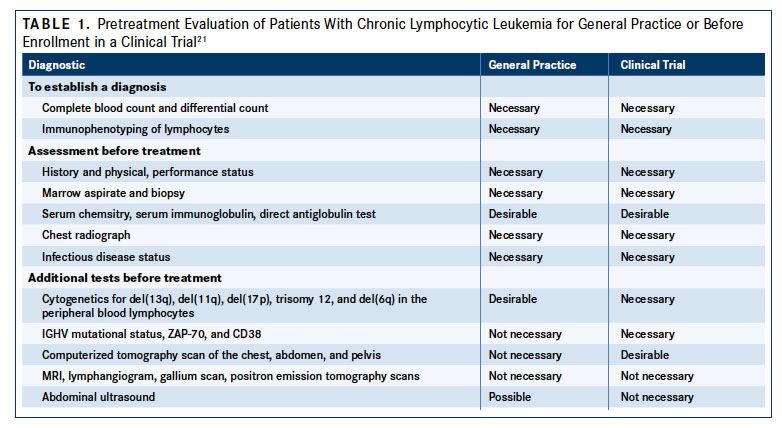
The risk for CLL increases with age (FIGURE 27). More than 70% of patients are older than 65 years at diagnosis, the median age at diagnosis is 72 years, and the incidence rate increases to >30:100,000/year at an age of more than 80 years.2Men are at an approximately 2-fold higher risk for developing CLL than women (male:female ratio of 1.9).6A genetic contribution to CLL susceptibility has been clearly established. Family members of patients with CLL have an 8.5-fold increased risk for developing the disease compared with the general population.9,10Genome-wide association studies have identified polymorphisms in more than 25 loci associated with familial CLL, including candidate genes involved in B-cell biology, apoptotic pathways, and regulatory microRNAs that may contribute to disease development.1114
Studies in veterans have established exposure to Agent Orange as an environmental risk factor for CLL.15Insecticide exposure and farming history have also been associated with a higher risk for developing CLL,16whereas blood transfusions and ionizing radiation have not.17,18
The precursor state to CLL, characterized as monoclonal B-cell lymphocytosis (MBL), is defined by the presence of less than 5000 monoclonal B cells, in the absence of lymphadenopathy, organomegaly, cytopenia, and clinical symptoms.2,19
Risk factors for MBL include increasing age and genetic disposition, including overlapping polymorphisms with those identified in CLL.19Population studies have shown that MBL is characterized by a bimodal distribution of clonal B cell counts that can be categorized as low-count and high-count MBL (less than or equal to or greater than 0.5x109/L clonal B cells, respectively). Low-count MBL rarely progresses to CLL, whereas the annual rate of progression from high-count MBL to CLL requiring therapy is 1% to 2%.20
EVOLVING METHODS OF CLL DIAGNOSIS
Many patients with CLL are asymptomatic and diagnosis follows the detection of lymphocytosis in a routine complete blood count (CBC), ie, above the normal adult upper limit of approximately 3500 cells per μL, typically ≥10,000 cells per μL.1If disease symptoms are present, they may include fatigue, involuntary weight loss, excessive night sweats, abdominal fullness with early satiety, increased frequency of infections, and symptoms of an autoimmune cytopenia, as well as enlarged lymph nodes, hepatomegaly, and splenomegaly, which can be detected by palpation.1
Essential components of the CLL diagnosis include blood immunophenotyping by flow cytometry and fluorescence in situ hybridization (FISH), lymph node biopsy if a peripheral analysis is not sufficient, and absolute B monoclonal B lymphocyte counts.3The initial workup should include a physical exam with palpation of node-bearing areas, determination of liver and spleen size, performance status, complete blood count, differential blood counts, platelets, and a metabolic panel.3Immunophenotyping by flow cytometry is required to establish CLL diagnosis based on cell identity, clonality, and quantity (<5x109/L peripheral CLL cells).2,3CLL cells co-express CD5, CD19, and CD23 antigens, weakly express CD20, CD79b, and surface immunoglobulin, with each clone restricted to expression of either kappa or lambda immunoglobulin light chains, and are negative for cyclin D1. Flow cytometric analysis for these markers is therefore used to establish a differential diagnosis from other B-cell lymphoproliferative disorders such as marginal zone lymphoma, lymphoplasmacytic lymphoma, and mantle cell lymphoma (MCL), all of which express B-cell surface antigens, but usually do not express CD23 and have negative or low CD43 expression.2,3MCL cells express CD5 but also exhibit enhanced expression of the gene encoding cyclin D1 (CCND1) due to the (11;14) translocation (t[11;14]), which is absent from CLL cells.1 As such, FISH analysis for t(11;14) can help to distinguish MCL from CLL.
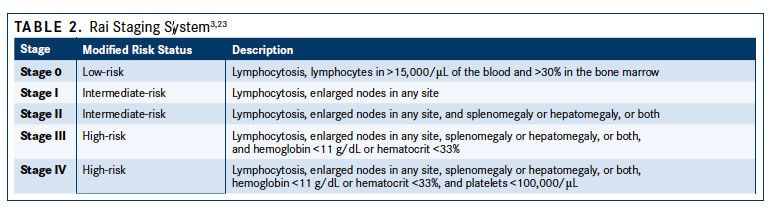
CLL cells in blood smears are small, mature lymphocytes with a narrow rim of cytoplasm and a dense nucleus without detectable nucleoli and with partially aggregated chromatin. Smudged cells, also known as Gumprecht nuclear shadows, are also morphologically characteristic for CLL.4Larger, atypical lymphocytes, cleaved cells, or prolymphocytes may be seen, but if the latter exceed 55%, prolymphocytic leukemia needs to be considered.1,21Bone marrow biopsy is not considered essential for the diagnostic workup in CLL but may be needed if immunophenotyping results are unclear. According to the International Workshop of Chronic Lymphocytic Leukemia (iwCLL) 2008 guidelines for the diagnosis and treatment of patients with CLL, a bone marrow asipirate and biopsy may be desirable in both practice and prior to enrollment in a clinical trial (TABLE 121). If a biopsy is performed, 4 different patterns of infiltration may be observed, which are nodular, interstitial, mixed nodular/interstitial, or diffuse (advanced disease).1Imaging using computed tomography (CT) scans can be beneficial for assessing the tumor load, monitoring disease progression, and determining the effects of investigational treatments in clinical trials, but it’s not recommended in asymptomatic patients or for staging.2,3
More than 80% of patients with previously untreated CLL have cytogenetic abnormalities, most commonly a deletion in chromosome 13q14.3(del[13q]; 55%), followed by del(11q) (18%), trisomy 12 (16%), and (del[17p]) (7%), and del(6q) (6%).22Recommended analyses include interphase cytogenetic analysis with FISH for the detection of the del(17p), which affects p53 expression, and if negative, molecular genetics is recommended for the detection of a TP53 mutation.2,3While FISH testing for cytogenetic abnormalities is suggested for pretreatment evaluation by the iwCLL 2008 guidelines, the guidelines especially recommend these tests in patients being enrolled in clinical trials.21Molecular genetic analysis should also include mutational status of the immunoglobulin heavy chain variable region gene (IGHV). Additional analyses considered informative for prognosis and treatment selection are stimulated metaphase analyses to identify complex karyotypes, extended FISH for additional cytogenetic abnormalities, and polymerase chain reaction or sequencing for mutations in NOTCH1, SF3B1, TP53, or MYD88.2,3
CLL STAGING Two clinical staging systems, the Rai and Binet systems, are used predominantly in the United States and Europe, respectively, to group patients with CLL into broad prognostic groups.23,24Both systems combine the presence of specific physical parameters, such as lymph node involvement, enlarged spleen and/or liver and blood parameters, including anemia or thrombocytopenia, to determine tumor burden. The Rai system, originally including 5 groups, has been modified to define low-risk disease (former stage 0) as lymphocytosis with leukemia cells in the blood and/or marrow. Intermediate risk disease (stage I/II) is defined as enlarged nodes in any site, and splenomegaly and/or hepatomegaly (lymph nodes being palpable or not), and high-risk disease (stage II/IV) as lymphocytosis and cytopenia (hemoglobin [Hb] level less than 11 g/dL and/ or platelet count of less than 100,000/μL) (TABLE 23,23).
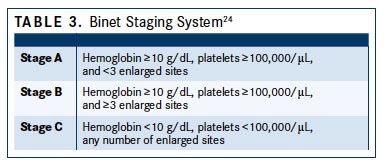
The Binet staging system relies on determining the number of involved areas, ie, enlarged lymph nodes of greater than 1 cm in diameter or organomegaly, and the presence anemia or thrombocytopenia. Binet stages are low risk (stage A), with less than 3 palpable enlarged sites without cytopenia; intermediate risk (stage B), with 3 or more palpable enlarged sites without cytopenia, and high risk (stage C) in all patients who have Hb of less than 10 g/dL and/or a platelet count of less than 100,000/μL, irrespective of organomegaly (TABLE 324).
Available survival estimates linked with staging suggest similar survival to age-matched controls for patients with lowrisk disease by Rai stage (median, 150 months), shorter survival for patients with intermediate-risk disease (median, 71- 101 months), and poor survival for high-risk features (median, 19 months).1,3However, these estimates reflect treatment with chemotherapy or chemoimmunotherapy, and life expectancies are increasing with newer small molecule inhibitor-based therapy.1,3
PROGNOSTIC MARKERS IN CLL
Newly diagnosed CLL is characterized by a highly variable clinical course, ranging from absence of symptoms for decades to the rapid development of symptoms or features of high-risk disease. Prognostic factors used for patient stratification include patient factors and clinical features of the disease, and genetic, molecular, and biochemical characteristics of the CLL clone.3
In addition to staging and lymphocyte doubling time, traditional prognostic factors or clinical features associated with poorer outcome are male sex, ≥65 years, poor performance status due to medical comorbidities, high serum levels of beta-2 microglobulin (>3.5 mg/L), high absolute lymphocyte count (>50,000 cells/μL), and/or late-stage disease at diagnosis.2527Elevated serum β2 microglobulin is an independent prognostic indicator for treatment-free interval, response to treatment, and overall survival (OS) in response to first-line chemoimmunotherapy regimens,3,26andif remaining after 6 months of treatment—for inferior progression- free survival (PFS) with ibrutinib (Imbruvica)-based therapies.28The use of these diagnostic tests is encouraged by current treatment guidelines and managed care companies. John L. Fox, MD, MHA, the vice president of medical affairs of Priority Health, explained in an interview with the American Journal of Managed Care®, “The cost of the diagnostics themselves are really not an issue. Those are the upfront costs in trying to establish what the most appropriate therapy is for that particular patient.”29
Biological prognostic markers that reflect CLL cell characteristics and are used for risk stratification include cytogenetic abnormalities; IGHV mutational status; TP53 mutation; expression of ZAP-70, CD49d (also known as integrin alpha-4), or CD38; and mutations in NOTCH1, SF3B1, BIRC3, and MYD88.3Among these, del(17p) and TP53 mutations are considered pertinent for treatment selection, and multiple other markers are considered of predictive value. Del(17p), which causes the loss of 1 TP53 allele and is associated with inactivating mutations in the other allele in 80% of patients with CLL, is considered the most important prognostic marker in CLL, with predictive value on treatment. The presence of del(17p) and/or mutations in TP53 defines a group of patients with CLL not eligible for chemoimmunotherapy regimens due to poor outcomes.22,30Targeted inhibitors of the B-cell receptor pathway and apoptosis inhibitors can produce high response rates in patients with del(17p) and are the preferred regimens in the first and subsequent lines of therapy.3The deletion is relatively rare at diagnosis (approximately 7%)22but frequent in relapsed/refractory disease, indicating acquisition or expansion of del(17q)-harboring CLL clones during treatment.31
Del(11q) has been linked to extensive lymphadenopathy, aggressive clinical course, and shorter median survival with traditional regimens (79 months).22However, the presence of del(11q) is not an adverse prognostic marker for response for treatment with ibrutinib.32
Among cytogenetic abnormalities, del(13q) is the only one that confers a favorable prognosis and long median survival (133 months).22Trisomy 12 has been linked to shorter survival.
Unmutated IGHV (defined as ≥98% homology with the germline gene sequence) reflects CLL originating from B cells that have not undergone a somatic mutation. The presence of unmutated IGHV predicts a more aggressive disease type and has traditionally been associated with significantly decreased survival compared with mutated IGHV, irrespective of disease stage.33,34Recent analyses have confirmed unmutated IGHV as a predictor of shorter survival with chemoimmunotherapy regimens including fludarabine and rituximab (Rituxan; FR), and fludarabine, chlorambucil, and rituximab (FCR).35,36In patients with mutated IGHV, FCR was associated with an improved survival in all cytogenetic groups except del(17p).36Unmutated IGHV does not predict adverse outcomes with ibrutinib-based regimens.37
Among prognostic surface markers detected by flow cytometry or immunohistochemistryincluding CD38, CD49d, and ZAP-70—CD49d is independent of FISH and IGHV.38 Multiple studies have associated CD38 and/or ZAP-70 expression with shorter PFS and OS, but despite positive correlations with the presence of unmutated IGHV, these markers are not considered appropriate surrogate markers—the latter due to discordance and variable expression during the course of disease.3Expression of ZAP-70 may be a stronger predictor of time until treatment is needed than IGHV mutational status or CD38 levels;39,40however, standardized detection of this nuclear marker is hampered by technical issues and it’s use is not yet recommended outside clinical trials.3
NOTCH1, SF3B1, and BIRC3 mutations are observed in approximately 4% to 15% of patients with newly diagnosed CLL, and 15% to 25% of patients with CLL refractory to fludarabine.3These mutations have been associated with varying prognostic significance across studies, such that their impact on treatment selection remains to be established, particularly for targeted agents.
Del(17p) and TP53 mutations are currently the only diseasebased predictive markers that affect treatment selection in CLL. With changing treatment options, particularly the inclusion of novel effective agents that prolong survival and have activity among patients considered high-risk in the era of chemotherapy-based regimens, the value of other prognostic markers continues to evolve.
As Steven E. Coutre, MD, professor of medicine (hematology) at Stanford University Medical Center, summarized in an interview: “A FISH test from the peripheral blood identifies patients who are at higher risk of progression and, in some cases like with del(17p), identifies patients who respond differently to different therapies. And so, those are the kinds of insights that we’re looking for to help us better choose treatments for our patients. And I think that’s where the field is going. Hopefully, we’ll be able to better tailor the choices we have toward specific patient groups.”41
References:
- Kipps TJ, Stevenson FK, Wu CJ, et al. Chronic lymphocytic leukaemia [published correction appears in Nat Rev Dis Primers. 2017;3:17008. doi:10.1038/nrdp.2017.8]. Nat Rev Dis Primers. 2017;3:16096. doi: 10.1038/nrdp.2016.96.
- Eichhorst B, Robak T, Montserrat E et al. Chronic lymphocytic leukaemia: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2015;26(suppl 5):v78-v84. doi: 10.1093/annonc/mdv303.
- National Comprehensive Cancer Network (NCCN). NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines): Chronic Lymphocytic Leukemia/Small Lymphocytic Lymphoma. Version 2.2017. NCCN website. www.nccn.org/professionals/physician_gls/ pdf/cll.pdf. Published February 21, 2017. Accessed May 15, 2017.
- Scarfò L, Ferreri AJ, Ghia P. Chronic lymphocytic leukaemia. Crit Rev Oncol Hematol. 2016;104:169-182. doi: 10.1016/j.critrevonc.2016.06.003.
- Pan JW, Cook LS, Schwartz SM, Weis NS. Incidence of leukemia in Asian migrants to the United States and their descendants. Cancer Causes Control. 2002;13(9):791-795.
- Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. CA Cancer J Clin 2017;67(1):7-30. doi: 10.3322/caac.21387.
- National Cancer Institute. Cancer stat facts: Chronic lymphocytic leukemia (CLL). NCI website. seer.cancer.gov/statfacts/html/clyl.html. Accessed May 15, 2017.
- Li Y, Wang Y, Wang Z, Yi D, Ma S. Racial differences in three major NHL subtypes: descriptive epidemiology. Cancer Epidemiol. 2015;39(1):8-13. doi: 10.1016/j.canep.2014.12.001.
- Goldin LR, Björkholm M, Kristinsson SY, Turesson I, Landgren O. Elevated risk of chronic lymphocytic leukemia and other indolent non-Hodgkin’s lymphomas among relatives of patients with chronic lymphocytic leukemia. Haematologica. 2009;94(5):647-653. doi: 10.3324/haematol.2008.003632.
- Cerhan JR, Slager SL. Familial predisposition and genetic risk factors for lymphoma. Blood. 2015;126(20):2265-2273. doi: 10.1182/blood-2015-04-537498.
- Di Bernardo MC, Crowther-Swanepoel D, Broderick P, et al. A genome-wide association study identifies six susceptibility loci for chronic lymphocytic leukemia. Nat Genet. 2008;40(10):1204-1210. doi: 10.1038/ng.219.
- Crowther-Swanepoel D, Broderick P, Di Bernardo MC, et al. Common variants at 2q37.3, 8q24.21, 15q21.3 and 16q24.1 influence chronic lymphocytic leukemia risk. Nat Genet. 2010;42(2):132-136. doi: 10.1038/ng.510.
- Berndt SI, Skibola CF, Joseph V, et al. Genome-wide association study identifies multiple risk loci for chronic lymphocytic leukemia. Nat Genet. 2013;45(8):868-876. doi: 10.1038/ ng.2652.
- Speedy HE, Di Bernardo MC, Sava GP, et al. A genome-wide association study identifies multiple susceptibility loci for chronic lymphocytic leukemia. Nat Genet. 2014;46(1):56- 60. doi: 10.1038/ng.2843.
- Baumann Kreuziger LM, Tarchand G, Morrison VA. The impact of Agent Orange exposure on presentation and prognosis of patients with chronic lymphocytic leukemia. Leuk Lymphoma. 2014;55(1):63-66. doi: 10.3109/10428194.2013.794267.
- Schinasi LH, De Roos AJ, Ray RM, et al. Insecticide exposure and farm history in relation to risk of lymphomas and leukemias in the Women’s Health Initiative observational study cohort. Ann Epidemiol. 2015;25(11):803-810. doi: 10.1016/j.annepidem. 2015.08.002.
- Hjalgrim H, Rostgaard K, Vasan SK, et al. No evidence of transmission of chronic lymphocytic leukemia through blood transfusion. Blood. 2015;126(17):2059-2061. doi: 10.1182/blood-2015-03-632844.
- Radivoyevitch T, Sachs RK, Gale RP, Smith MR, Hill BT. Ionizing radiation exposures in treatments of solid neoplasms are not associated with subsequent increased risks of chronic lymphocytic leukemia. Leuk Res. 2016;43:9-12. doi: 10.1016/j.leukres. 2016.02.008.
- Strati P, Shanafelt TD. Monoclonal B-cell lymphocytosis and early-stage chronic lymphocytic leukemia: diagnosis, natural history, and risk stratification. Blood. 2015;126(4):454-462. doi: 10.1182/blood-2015-02-585059.
- Rawstron AC, Bennett FL, O’Connor SJ, et al. Monoclonal B-cell lymphocytosis and chronic lymphocytic leukemia. N Engl J Med. 2008;359(6):575-583. doi: 10.1056/ NEJMoa075290.
- Hallek M, Cheson BD, Catovsky D, et al. Guidelines for the diagnosis and treatment of chronic lymphocytic leukemia: a report from the International Workshop on Chronic Lymphocytic Leukemia updating the National Cancer InstituteWorking Group 1996 guidelines [erratum published in Blood. 2018;112(13):5259. doi: 10.1182/blood-2008-10-186254.]. Blood. 2008;111(12):5446-5456. doi: 10.1182/ blood-2007-06-093906.
- Döhner H, Stilgenbauer S, Benner A et al. Genomic aberrations and survival in chronic lymphocytic leukemia. N Engl J Med. 2000;343(26):1910-1916. doi: 10.1056/ NEJM200012283432602.

Peers Discuss Role of Pola-R-CHP vs R-CHOP in Newly Diagnosed DLBCL
April 19th 2024During a Case-Based Roundtable® event, Haifaa Abdulhaq, MD discussed with participants whether the POLARIX trial influences their choice to use the pola-R-CHP as opposed to R-CHOP regimen for patients with newly diagnosed diffuse large B-cell lymphoma.
Read More
Powell Reviews Updated IO/TKI Data and AE Management in Endometrial Cancer
April 18th 2024During a Case-Based Roundtable® event, Matthew A. Powell, MD, discussed the case of a patient with advanced endometrial cancer treated with lenvatinib plus pembrolizumab who experienced grade 2 treatment-related hypertension.
Read More
Savona Discusses First-Line JAK Inhibition for Patients With Myelofibrosis at Risk of Anemia
April 17th 2024During a Case-Based Roundtable® event, Michael Savona, MD, and participants discussed the case of a patient with myelofibrosis and moderate anemia receiving JAK inhibitor therapy.
Read More
Biomarker Testing Paves the Way for Better Targeted Therapies in NSCLC
April 16th 2024At a live virtual event, Edward S. Kim, MD, MBA, discussed the evolving landscape of biomarker testing before making treatment decisions for patients with early-stage non–small cell lung cancer (NSCLC).
Read More