Managing Toxicities in Targeted Therapies for Metastatic Melanoma
Although single-target inhibitors and have demonstrated significant clinical benefit, the toxicities are not limited to cancer cells alone and associated toxicities and adverse events are often observed.
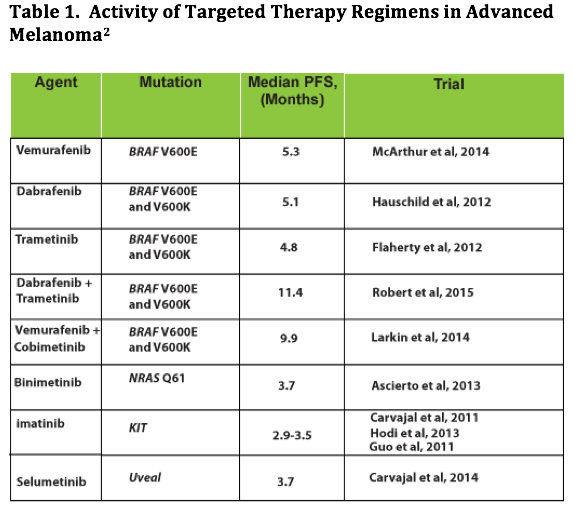
MAP kinase pathway activation due to mutations in theBRAF,MEK,NRAS, andKitgenes is observed in majority of patients with advanced melanoma. Inhibition of the activated pathway slows disease progression. Abundant clinical data availability supporting the effectiveness of these targeted therapies in controlling tumor progression in patients with melanoma who have activating mutations has led to approval of several inhibitors ofBRAF,MEK,NRAS, andKitgenes. These include FDA-approved BRAF inhibitors (BRAFi) vemurafenib and dabrafenib, MEK inhibitors (MEKi) trametinib, and KIT inhibitor imatinib (Table 1).1,2
Although single-target inhibitors and have demonstrated significant clinical benefit, the effects are not limited to cancer cells alone and associated toxicities and adverse events (AEs) are often observed. Although the AEs are mild to moderate and rarely life threatening, the therapies present characteristic patterns of drug-related toxicities. Optimal use to maximize their therapeutic potential therefore depends on efficient side-effect management. Some of the common reported AEs and their management strategies from various studies are reported here.
Common BRAF and MEK Inhibitors and Associated Toxicities
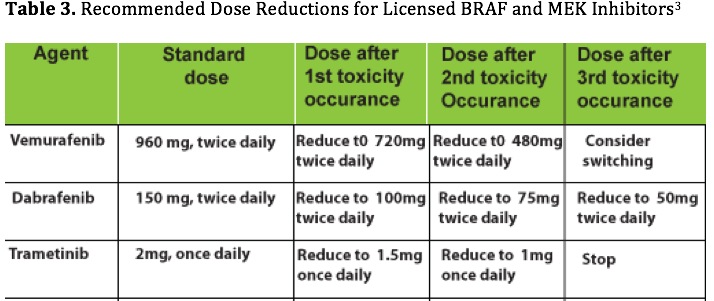
The majority of patients on these inhibitors experience (>90%) experience AEs; however, these are generally mild to moderate (Common Toxicity Criteria of Adverse Events, CTCAE v4.0, grade 1-2).3The basic recommendations for managing the general AEs and common toxicities associated with targeted inhibitors are summarized inTables 2 and 3.4The management based on specific drugs is also presented along with recommendations for toxicity management.

Vemurafenib
The most common AEs with vemurafenib treatment are rash (49%), arthralgia (39%), fatigue (34%), photosensitivity (31%), alopecia (26%) and nausea (19%). 46% patients have experienced grade 3 or 4 AEs, which are most commonly cutaneous SCC (12%), rash (5%), liver function abnormalities (5%), arthralgia (3%) and fatigue (3%). Vemurafenib side effects appear in a predictable pattern: photosensitivity and rash occur usually within days of starting the drug. Arthralgia, diarrhea, fatigue, alopecia and occurrence of other skin lesions tend to occur over weeks and months. While photosensitivity continues with drug use, other AEs including rash, skin lesions and arthralgia can regress and be less problematic after the first few months.
Dabrafenib
The most common dabrafenib-induced AEs are hyperkeratosis (39%), headache (35%), arthralgia (35%), and pyrexia (32%). Other skin toxicities included keratocanthoma and SCC (10%), but unlike vemurafenib, photosensitivity is extremely rare. Grade 3 to 4 AEs are uncommon, but pyrexia was identified as a new and potentially serious toxicity occurring in some 5% of treated patients, not previously seen with vemurafenib.
Trametinib
Rash, diarrhea, fatigue, peripheral edema, and acneiform dermatitis are the most common side effects observed after treatment with trametinib. Cardiac (decreased ejection fraction or ventricular dysfunction), ocular and interstitial lung disease (ILD) or pneumonitis events are also reported in 7%, 9%, and 2% of patients treated in the METRIC trial, which are not recognized side effects of BRAFi. However, secondary skin neoplasms do not occur with the MEKi.
Dabrafenib and trametinib
The combination regimen of dabrafenib and trametinib using full doses of both agents appears to generate fewer skin AEs compared with BRAFi monotherapy. AEs that occur more frequently with combination therapy are fever (51%) and chills (30%), fatigue (35%), diarrhea (24%), hypertension (22%), and vomiting (20%). Alopecia is reported less often in the combination arm
Recommendations for Managing Toxicities
Skin toxicities
Rash, photosensitivity, pruritis, dry skin, papilloma, alopecia, keratoacanthoma, and SCC are the signature of BRAFi monotherapy.
Photosensitivity with vemurafenib:
- Routine use of sunscreen with sun protection factor of at least 30
Diarrhea
- To be managed symptomatically on an outpatient basis
- Immediate initiation of loperamide, dose interruption, and lower dose retreatment initiation
- Dietary modifications for patients continuing on treatment with tolerable chronic loose bowel motions, including eating small frequent meals, introduction of a BRAT diet (bananas, rice, apples, and toast)
- No consumption of lactose-containing products and maintaining fluid intake with electrolyte replacement
Pyrexia
Specifically associated with dabrafenib rather than vemurafenib treatment.
- Exclude sepsis in patients with pyrexia
- Patient education and supportive care
- When accompanied by hypotension or general malaise, may require patient hospitalization.
- Unwell patients who are pyrexial but not septic, dose interruption, use of paracetamol or low-dose prednisolone; allow reintroduction of drug on recovery
- Patients with intermittent fever pattern due to their chronic therapy without complicated factors or sepsis:remain at home and manage their fever with paracetamol, steroids, and/or temporary drug dose interruption
Arthralgia
Severity of arthralgia is probably higher with vemurafenib compared with dabrafenib: 56% versus 35% incidence overall.
- Administration of analgesics and antiinflammatory drugs improves simple arthralgia
- Severe arthralgia: dose interruption, nonsteroidal antiinflammatory drugs, and low-dose corticosteroids; followed by reintroduction of vemurafenib at a lower dose.
Fatigue
Observed with use of many oral kinase inhibitors.
- Chronic fatigue managed with dose modifications or low-dose steroids
Cardiac complications
- Dabrafenib or vemurafenib not recommended in patients with uncorrectable electrolyte abnormalities (including low magnesium), long QT syndrome, or those who are taking medicinal products known to prolong the QT interval
- Initiation of treatment with dabrafenib or vemurafenib is also not recommended in patients with QTc greater than 500 ms
- Close monitoring recommended in patients with moderate to severe hepatic impairment, which may impact drug metabolism
Ophthalmologic complications
Mostly observed during treatment with MEKi.
- Managed with temporary dose interruption, ophthalmology review, a course of topical steroids, and in most cases, dose reduction
- Stop trametinib in patients with blurred vision, loss of vision, or color spots. Require urgent ophthalmologic review, and if symptoms abate, in the absence of RVO, can be retreated with a lower drug dose.
- Prior to initiating MEKi treatment, a risk assessment should be undertaken and treatment avoided in patients with preexisting ocular conditions such as glaucoma.
Targeted therapies have revolutionized treatment options for patients with metastatic melanoma. However, like other targeted therapies, acute and chronic exposure to these drugs are associated with predictable patterns of AEs. Algorithms have been designed to assist in the management of the more common and serious AEs. Use of these along with high-quality patient education and support can help in avoiding drug discontinuation. This maximizes the opportunity for patients to benefit from prolonged disease control.
References
- UpToDate. Sosman JA. Molecularly targeted therapy for metastatic melanoma.http://www.uptodate.com/contents/molecularly-targeted-therapy-for-metastatic-melanoma?source=see_link. Accessed November 2, 2015.
- Johnson D B and Sosman JA. Therapeutic advances and treatment options in metastatic melanoma.JAMA Oncol. 2015;1(3):380-386.
- Flaherty L, Hamid O, Linette G, et al. A single-arm, open-label, expanded access study of vemurafenib in patients with metastatic melanoma in the United States.Cancer J2014;20:18-24.
- Welsh SJ and Corrie PG. Management of BRAF and MEK inhibitor toxicities in patients with metastatic melanoma.Ther Adv Med Oncol2015;7(2):122-136.

Savona Discusses First-Line JAK Inhibition for Patients With Myelofibrosis at Risk of Anemia
April 17th 2024During a Case-Based Roundtable® event, Michael Savona, MD, and participants discussed the case of a patient with myelofibrosis and moderate anemia receiving JAK inhibitor therapy.
Read More