Safety and Efficacy of Combination Targeted Therapy and Radiotherapy
A review of currently published clinical trials examining the combinaiton of radiation therapy with commonly used targeted agents, such as vascular endothelial growth factor inhibitors, endothelial growth factor receptor inhibitors, and inhibitors of the PI3K/Akt/mTOR pathway.
Abstract
Targeted cancer therapies that act on specific drivers of oncogenesis are rapidly entering clinical use. While many of these agents are ineffective at improving cure rates as monotherapy, there is ample preclinical evidence that they are both chemosensitizing and radiosensitizing, and can improve cure rates when utilized in combination treatment regimens. There is therefore a need for high-quality safety and efficacy data on targeted therapy in combination with radiation therapy (RT). This article reviews the currently published clinical trials examining the combination of RT with commonly used targeted agents, such as vascular endothelial growth factor inhibitors, endothelial growth factor receptor inhibitors, and inhibitors of the PI3K/Akt/mTOR pathway. Continued efforts to develop high-quality clinical trial data combining targeted agents with RT are necessary for patient safety and to improve clinical outcomes.
Introduction
1
2
3
Radiation therapy (RT), together with surgery and chemotherapy, is one of the primary modalities used in definitive and palliative cancer treatment. Utilization analyses have revealed that RT is a part of initial treatment in approximately 30% of patients with cancer,and approximately 50% of patients overall receive RT.In comparing contribution toward cure by treatment modality, a European Union expert panel determined that cure is achieved in 49% of patients by surgery, 40% by RT, and 11% by chemotherapy.Given these statistics and in light of advancements expanding the clinical indications of RT, RT will continue to be an essential modality in the treatment of malignancies in the future.
4,5
Targeted cancer agents that block specific molecular pathways involved in oncogenesis are rapidly shifting the landscape of cancer treatment. While these agents present promising opportunities for the treatment of many malignancies, the majority are cytostatic, and many impart modest, if any, survival benefit as monotherapy.However, there is preclinical evidence that these agents are radiosensitizing and may improve cure rates when used in combination with RT.
6-18
The radiosensitizing effects of classical chemotherapeutics, including cisplatin, 5-fluorouracil (5-FU), taxanes, and temozolomide, have been well characterized, and the combination of such agents with RT has been demonstrated to improve survival and cure rates across many cancer types in randomized clinical trials.Since these agents are nonspecific and radiosensitize normal tissue, such treatment carries greater toxicity. While this toxicity is accepted due to the even greater clinical benefit, targeted agents present an exciting opportunity because they may selectively radiosensitize tumor cells without a concomitant increase in normal tissue toxicity. In this review, we summarize the currently published clinical trials of commonly used therapies in combination with RT, with attention to data on efficacy and toxicity.
Hormone Therapy
17
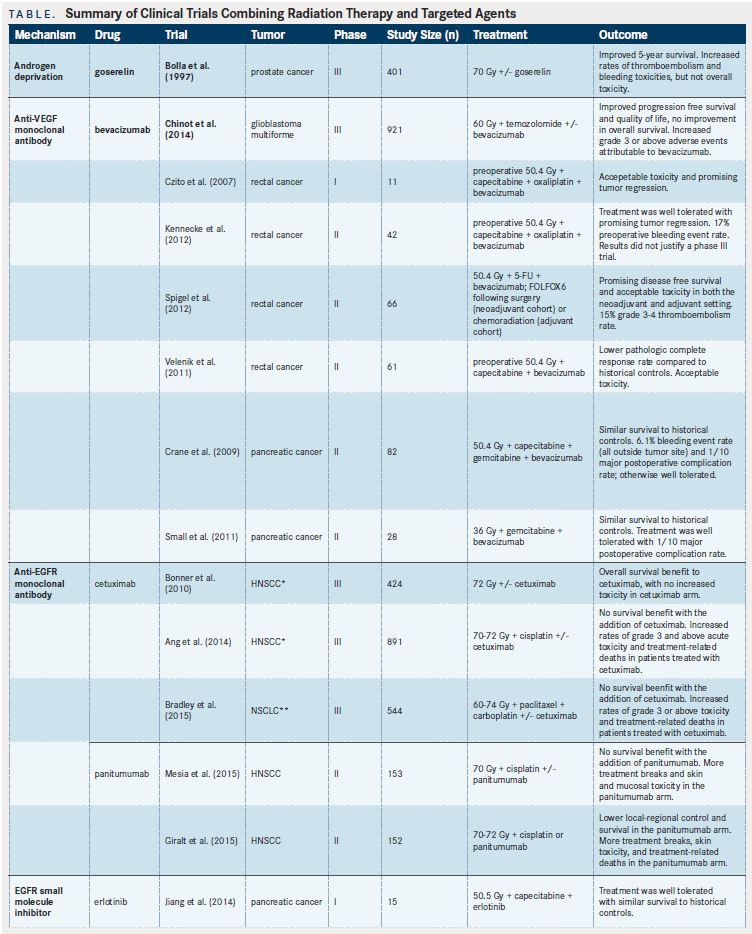
Androgen-deprivation therapy (ADT) in combination with RT for prostate cancer can be viewed as an early targeted biologic approach. In 1997, the seminal Southwest Oncology Group (SWOG)/European Organization for Research and Treatment of Cancer (EORTC) randomized trial demonstrated improved survival with the addition of goserelin to definitive RT for locally advanced prostate cancer.Grade 3 or above acute and late toxicities were not significantly different with the addition of ADT. However, combined late grade 1-3 toxicities, including urinary incontinence and urethral stricture, were significantly increased in patients treated with ADT. Despite the higher rate of adverse effects (AEs) of combined therapy, this toxicity was deemed acceptable, and ADT with RT is currently an accepted standard of care for locally advanced prostate cancer.
Monoclonal Antibodies
19
20
21-24
Bevacizumab, a monoclonal antibody against vascular endothelial growth factor A (VEGF-A), is a pioneering targeted agent that has been studied in large clinical trials.A recently published phase III trial utilizing bevacizumab with temozolamide and RT in glioblastoma multiforme improved progression- free survival (PFS) and quality-of-life endpoints, but not overall survival (OS).The rates of grade 3 AEs were increased with the addition of bevacizumab. Interestingly, these toxicities were not primarily radiation-related. Instead, the majority were attributable to bevacizumab, and included thromboembolic events, bleeding events, impaired wound healing, gastrointestinal (GI) perforation, and congenital heart failure. Specifically, the rate of cerebral hemorrhage was increased in patients treated with bevacizumab compared with placebo (3.3% vs 2.0%). In rectal cancer, several early trials demonstrated the feasibility of using bevacizumab in combination with chemoradiation, with overall similar rates of AEs compared with historical controls.However, increased GI bleeding thought to be due to the addition of bevacizumab was also observed in these studies.
23
25,26
For example, a phase II study from Canada reported severe preoperative bleeding events in 17% of patients treated with combination bevacizumab and chemoradiation.In pancreatic cancer, two phase II trials evaluating the addition of bevacizumab to chemoradiation did not improve survival outcomes compared with historical rates.Several bleeding events were noted with the addition of bevacizumab, but the sites of bleeding were outside of the radiation field. Ultimately, further studies are needed to determine the safety and efficacy of bevacizumab with chemoradiation and its application in the treatment of malignancies. Nevertheless, the data appear to support acceptable, though perhaps increased, toxicities of bleeding and thromboembolic events attributable specifically to bevacizumab.
27
28
29
Activating mutations of the EGFR/PI3K/Akt/ mTOR pathway are common in cancers and have been implicated in radioresistance. The epidermal growth factor receptor (EGFR) inhibitor cetuximab has been found to have potent radiosensitizing properties in preclinical trials.In a large, multi-institutional, randomized trial, Bonner et alreported an OS benefit when adding cetuximab to RT in locally advanced head and neck squamous cell carcinoma (HNSCC). With the exception of acneiform rash and infusion reactions, the incidence of grade 3 toxicity did not differ significantly between patient arms. This trial demonstrated the feasibility, safety, and efficacy of adding cetuximab to RT. However, the follow- up study, RTOG 0522, which added cetuximab to cisplatin-based chemoradiation for locally advanced HNSCC, did not show a survival benefit with the addition of cetuximab.Indeed, patients who were treated with cetuximab in addition to cisplatin had more interruptions in RT, increased treatmentrelated death, and increased grade 3 AEs, including mucositis and anorexia.
30
Similarly, a recently reported randomized phase III trial for stage IIIA/B nonsmall-cell lung cancer (NSCLC), RTOG 0617, demonstrated no clinical benefit with the addition of cetuximab to standard or dose-escalated chemoradiation.However, patients receiving cetuximab in addition to chemoradiation experienced significantly higher rates of grade 3 toxicities compared with those receiving chemoradiation alone (86% vs 70%). Further, there were more treatment-related deaths with the use of cetuximab (4.2% vs 2.2%).
31
32
A related EGFR antibody, panitumumab, has been examined in the randomized phase II trials for HNSCC, CONCERT-1,and CONCERT-2.CONCERT-2 compared panitumumab plus RT to cisplatin-based chemoradiation in patients with locally advanced HNSCC. This study demonstrated inferior local control at 2 years in those receiving panitumumab (51% vs 61%). Toxicities were considered to be similar between the groups, with the exception of increased skin toxicity in the panitumumab group (24% vs 11%). CONCERT-1 examined panitumumab plus cisplatin-based chemoradiation compared with chemoradiation alone. This trial demonstrated no additional benefit with the addition of panitumumab. There were more treatment breaks and grade 3 AEs in the panitumumab arm, most commonly mucosal inflammation (55% vs 24%), radiation dermatitis (28% vs 13%), and dysphagia (39% vs 27%). There was one treatment-related death in each arm in this trial.
Small-Molecule Inhibitors
33
34
35
36
37
38
39
40
41
41
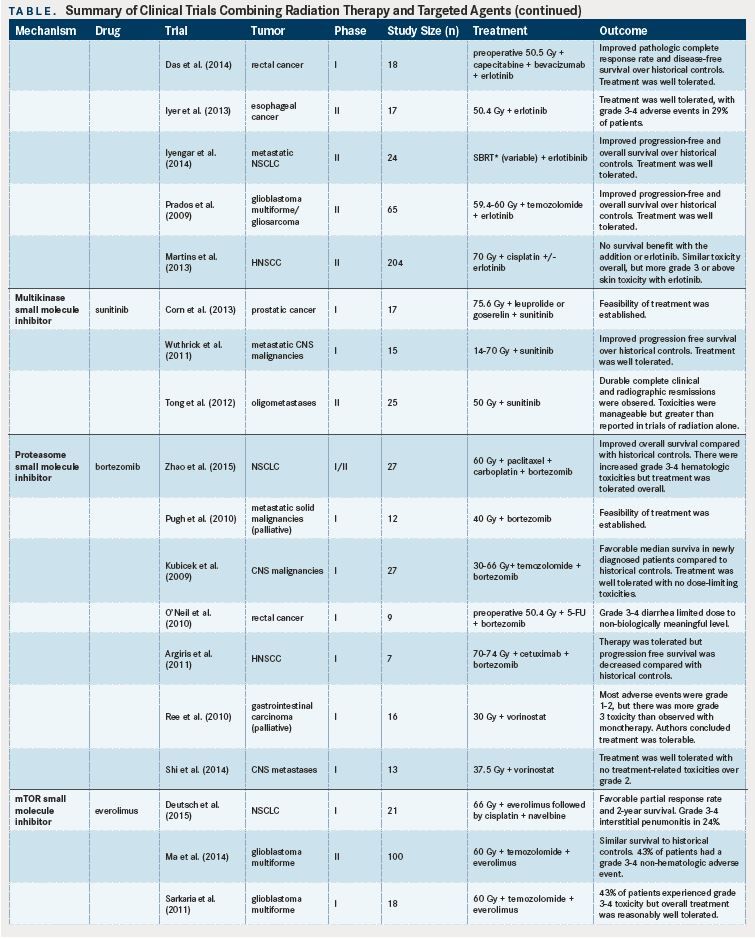
Erlotinib, an EGFR small-molecule inhibitor, has been shown to be well tolerated in combination with: capecitabine and RT in locally advanced pancreatic cancer; capecitabine, bevacizumab, and RT in rectal cancer; and RT in esophageal cancer.A phase II trial combining erlotinib with stereotactic body RT for patients with progressive metastatic NSCLC demonstrated improved PFS and OS compared with historical controls, and was well tolerated, with only two of 24 RT-related grade 3 toxicities.Similarly, a phase II trial of erlotinib combined with temozolomide in addition to RT in glioblastoma multiforme reported better survival than historical controls and an acceptable safety profile.However, a randomized phase II trial comparing erlotinib plus cisplatin-based chemoradiation with chemoradiation alone in patients with locally advanced HNSCC demonstrated no difference in clinical complete response rates between the two groups.The addition of erlotinib did not increase the rate of AEs overall, but patients receiving erlotinib experienced a higher rate of grade 3 rash (13% vs 2%). Sunitinib, a multikinase inhibitor, has been shown to be well tolerated when combined with ADT and RT in localized high-risk prostate cancer,in combination with RT for central nervous system (CNS) malignancies,and for oligometastatic disease,with good clinical responses in phase I and II studies.
42
43
44
45
46
47
The proteasome inhibitor bortezomib may radiosensitize tumors by blocking DNA repair and has been examined in several phase I and II trials.In a phase I/II trial examining the addition of bortezomib to carboplatin, paclitaxel, and RT for stage III NSCLC, patients tolerated treatment overall, but had more grade 3 or 4 hematologic toxicities.Similarly, a phase I trial looking at bortezomib concurrent with palliative RT in patients with metastatic solid cancers demonstrated feasability, with patients most commonly experiencing hematologic AEs.Another phase I trial examining bortezomib plus concurrent temozolomide and RT for CNS malignancies demonstrated safety, with no dose-limiting toxicities noted.Conversely, a phase I trial adding bortezomib to chemoradiation for rectal cancer demonstrated an increased rate of dose-limiting toxicities (defined as grade 3 diarrhea).This limited the maximum tolerated dose to the first dosage level of the study, which was not considered to be biologically meaningful. Interestingly, and as a note of caution in combining biologic therapies, a phase I trial combining bortezomib with cetuximab and RT in HNSCC showed increased rates of early progression of disease compared with cetuximab and radiation historical controls.Biologic correlates of the trial demonstrated enhanced EGFR prosurvival signaling with bortezomib, likely counteracting the therapeutic effect of cetuximab and RT.
48
49
50
Histone deacetylase (HDAC) inhibitors may also radiosensitize tumors via DNA repair inhibition,and have been examined in early-phase clinical trials. In the phase I PRAVO trial,which examined vorinostat in the context of palliative pelvic radiation, the majority of AEs were grade 1 or 2. Grade 3 or higher treatment-related toxicities of fatigue and anorexia were noted. The authors of this study concluded that vorinostat can be safely combined with palliative pelvic RT. A phase I trial demonstrated that the combination of vorinostat with whole-brain RT for brain metastases is safe and well tolerated, with no treatment-related toxicities above grade 2.Taken together, these findings are encouraging for the potential use of HDAC inhibitors in curative RT.
51
52
53
54
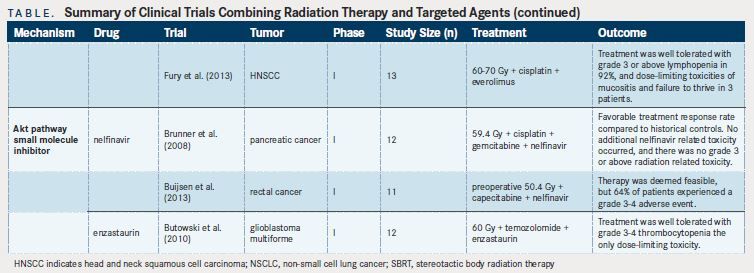
mTOR inhibitors have been shown to be potent radiosensitizers.One recent phase I trial combining everolimus with thoracic RT in NSCLC reported grade 3-4 interstitial pneumonitis in 5 of 21 patients.The authors concluded that pulmonary toxicities were of concern and should be carefully monitored. A phase II trial of everolimus, temozolomide, and RT in patients with glioblastoma multiforme did not demonstrate a survival benefit compared with historical controls, with “moderate toxicity” of 12% grade 4 nonhematologic toxicities and one treatment-related death out of the 100 patients enrolled to the study.A previous phase I study suggested that the dose-limiting toxicities, including fatigue, hematologic toxicity, and liver dysfunction, were not related to RT.In the context of patients with HNSCC, a phase I study of everolimus, cisplatin, and RT showed the combination to be well tolerated, with the expected dose-limiting toxicities of mucositis and failure to thrive in three patients.
55
56
57
The use of Akt pathway inhibitors also has been studied in limited clinical trials. A phase I trial of the Akt pathway inhibitor nelfinavir and chemoradiation for locally advanced pancreatic cancer demonstrated no grade 3 radiation-related AEs, and the addition of nelfinavir was felt to have acceptable toxicity and promising activity.Another phase I trial examined the safety of nelfinavir combined with chemoradiation for locally advanced rectal cancer.Of the 11 patients analyzed, three had grade 3 diarrhea, two had grade 3 transaminitis, one had grade 3 cholangitis, and one had a grade 4 postoperative wound infection. No dose-limiting toxicities were noted at the beginning dosage level, so this dosage was recommended for phase II trials. Finally, a phase I trial adding enzastaurin, a PKC- and PI3K/ Akt pathway inhibitor, to temozolomide and RT for glioblastoma multiforme showed good tolerance, with grade 3 thrombocytopenia in two patients being the only dose-limiting toxicity.
Immunomodulators
58,59
59,60
There are promising novel biologic rationales for combining immunomodulators with RT. Radiation may stimulate the immune response when combined with immunomodulators.In particular, checkpoint inhibitors such as anti-CTLA-4 and anti-PDL1/ PD-1 have shown great promise in combination with RT.Ongoing early clinical trials currently are studying combining RT and immunomodulators.
Conclusion
61,62
63
4
65
This review underscores an underrepresentation of RT in targeted therapy clinical trials, despite the common use of RT in cancer treatment. Approximately, 70 novel targeted cancer therapies are currently approved by the US Food and Drug Administration, with hundreds more in development. Many of these agents have been demonstrated in the preclinical setting to enhance the radiation effect; however, the majority have not been investigated in even phase I clinical trials for safety in the context of RT. The published clinical trials reviewed here suggest that it is feasible to combine targeted agents with RT. However, there are many single institutional case series that have provided mixed data, with some suggesting safety and others suggesting unexpected toxicities.One recent analysis demonstrated that of the over 400 phase I trials published yearly, only 30 include RT.There is clearly a deficit of high-quality clinical data to guide the care of patients with cancer who are treated with targeted agents, and who have an indication for RT. To begin to address the lack of combined RT phase I trials, guidelines have been published by the National Cancer Institute,64 Radiation Therapy Oncology Group,and National Cancer Research Instituteemphasizing the importance of such studies and considerations of trial design and optimization. Such development of clinical trial data will ensure safe patient care and optimization of combined therapy treatment strategies.
References:
- Smith BD, Haffty BG, Wilson LD, Smith GL, Patel AN, Buchholz TA. The future of radiation oncology in the United States from 2010 to 2020: will supply keep pace with demand?J Clin Oncol.2010;28(35):5160-5165.
- Delaney G, Jacob S, Featherstone C, Barton M. The role of radiotherapy in cancer treatment: estimating optimal utilization from a review of evidence-based clinical guidelines.Cancer.2005;104(6):1129-1137.
- Einhorn J FJ-E, Müller T, Lamnevik G, et al. Radiotherapy for Cancer. Vol 1. Acta Oncologica. 1996;35 (suppl. 6):1-100 and (suppl 7):1-152.
- Lawrence YR, Vikram B, Dignam JJ, et al. NCI-ROTG translational program strategic guidelines for the early-stage development of radiosensitizers.J Natl Cancer Inst.2013;105(1):11-24. doi: 10.1093/jnci/djs472.
- Fojo T, Parkinson DR. Biologically targeted cancer therapy and marginal benefits: are we making too much of too little or are we achieving too little by giving too much?Clin Cancer Res.2010;16(24):5972-5980. doi: 10.1158/1078-0432.CCR-10-1277.
- Wilson GD, Bentzen SM, Harari PM. Biologic basis for combining drugs with radiation.Semin Radiat Oncol.2006;16(1):2-9.
- Golden EB, Formenti SC, Schiff PB. Taxanes as radiosensitizers.Anticancer Drugs.2014;25(5):502-511. doi: 10.1097/CAD.0000000000000055.
- Stupp R, Mason WP, van den Bent MJ, et al; for the European Organisation for Research and Treatment of Cancer Brain Tumor and Radiotherapy Groups National Cancer Institute of Canada Clinical Trials Group. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma.N Engl J Med.2005;352(10):987-996.
- Pignon JP, le Maitre A, Maillard E, Bourhis J; MACH-NC Collaborative Group. Meta-analysis of chemotherapy in head and neck cancer (MACH-NC): an update on 93 randomised trials and 17,346 patients.Radiother Oncol.2009;92(1):4-14. doi: 10.1016/j.radonc.2009.04.014.
- Bonner JA, Harari PM, Giralt J, et al. Radiotherapy plus cetuximab for squamous-cell carcinoma of the head and neck.N Engl J Med.2006;354(6):567-578.
- Schaake-Koning C, van den Bogaert W, Dalesio O, et al. Effects of concomitant cisplatin and radiotherapy on inoperable non-small-cell lung cancer.N Engl J Med.1992;326(8):524-530.
- Cooper JS, Guo MD, Herskovic A, et al. Chemoradiotherapy of locally advanced esophageal cancer: long-term follow-up of a prospective randomized trial (RTOG 85-01). Radiation Therapy Oncology Group.JAMA.1999;281(17):1623-1627
- Smalley SR, Benedetti JK, Haller DG, et al. Updated analysis of SWOG-Directed Intergroup Study 0116: a phase III trial of adjuvant radiochemotherapy versus observation after curative gastric cancer resection.J Clin Oncol.2012;30(19):2327-2333. doi: 10.1200/JCO.2011.36.7136.
- Krook JE, Moertel CG, Gunderson LL, et al. Effective surgical adjuvant therapy for high-risk rectal carcinoma.N Engl J Med.1991;324(11):709-715.
- Epidermoid anal cancer: results from the UKCCCR randomised trial of radiotherapy alone versus radiotherapy, 5-fluorouracil, and mitomycin. UKCCCR Anal Cancer Trial Working Party. UK Co-ordinating Committee on Cancer Research.Lancet.1996;348(9034):1049-1054.
- Chemoradiotherapy for Cervical Cancer Meta-Analysis Collaboration. Reducing uncertainties about the effects of chemoradiotherapy for cervical cancer: a systematic review and meta-analysis of individual patient data from 18 randomized trials.J Clin Oncol.2008;26(35):5802-5812. doi: 10.1200/JCO.2008.16.4368.
- Bolla M, Gonzalez D, Warde P, et al. Improved survival in patients with locally advanced prostate cancer treated with radiotherapy and goserelin.N Engl J Med.1997;337(5):295-300.
- James ND, Hussain SA, Hall E, et al; BC2001 Investigators. Radiotherapy with or without chemotherapy in muscle-invasive bladder cancer.N Engl J Med.2012;366(10):1477-1488.
- Schmidt B, Lee HJ, Ryeom S, Yoon SS. Combining bevacizumab with radiation or chemoradiation for solid tumors: a review of the scientific rationale, and clinical trials.Curr Angiogenes.2012;1(3):169-179.
- Chinot OL, Wick W, Mason W, et al. Bevacizumab plus radiotherapy-temozolomide for newly diagnosed glioblastoma.N Engl J Med.2014;370(8):709-722. doi: 10.1056/NEJMoa1308345.
- Czito BG, Bendell JC, Willett CG, et al. Bevacizumab, oxaliplatin, and capecitabine with radiation therapy in rectal cancer: phase I trial results.Int J Radiat Oncol Biol Phys.2007;68(2):472-478.
- Spigel DR, Bendell JC, McCleod M, et al. Phase II study of bevacizumab and chemoradiation in the preoperative or adjuvant treatment of patients with stage II/III rectal cancer.Clin Colorectal Cancer.2012;11(1):45-52. doi: 10.1016/j.clcc.2011.04.002.
- Kennecke H, Berry S, Wong R, et al. Pre-operative bevacizumab, capecitabine, oxaliplatin and radiation among patients with locally advanced or low rectal cancer: a phase II trial.Eur J Cancer.2012;48(1):37-45. doi: 10.1016/j.ejca.2011.05.016.
- Velenik V, Ocvirk J, Music M, et al. Neoadjuvant capecitabine, radiotherapy, and bevacizumab (crab) in locally advanced rectal cancer: results of an open-label phase II study.Radiat Oncol.2011;6:105. doi: 10.1186/1748-717X-6-105.
- Crane CH, Winter K, Regine WF, et al. Phase II study of bevacizumab with concurrent capecitabine and radiation followed by maintenance gemcitabine and bevacizumab for locally advanced pancreatic cancer: radiation therapy oncology group rtog 0411.J Clin Oncol.2009;27(25):4096-4102. doi: 10.1200/JCO.2009.21.8529.
- Small W, Jr., Mulcahy MF, Rademaker A, et al. Phase II trial of full-dose gemcitabine and bevacizumab in combination with attenuated three-dimensional conformal radiotherapy in patients with localized pancreatic cancer.Int J Radiat Oncol Biol Phys.2011;80(2):476-482. doi: 10.1016/j.ijrobp.2010.02.030.
- Harding J, Burtness B. Cetuximab: an epidermal growth factor receptor chemeric human-murine monoclonal antibody.Drugs Today (Barc).2005;41(2):107-127.
- Bonner JA, Harari PM, Giralt J, et al. Radiotherapy plus cetuximab for locoregionally advanced head and neck cancer: 5-year survival data from a phase 3 randomised trial, and relation between cetuximab-induced rash and survival.Lancet Oncol.2010;11(1):21-28. doi: 10.1016/S1470-2045(09)70311-0.
- Ang KK, Zhang Q, Rosenthal DI, et al. Randomized phase III trial of concurrent accelerated radiation plus cisplatin with or without cetuximab for stage III to IV head and neck carcinoma: RTOG 0522.J Clin Oncol.2014;32(27):2940-2950.
- Bradley JD, Paulus R, Komaki R, et al. Standard-dose versus high-dose conformal radiotherapy with concurrent and consolidation carboplatin plus paclitaxel with or without cetuximab for patients with stage iiia or iiib non-small-cell lung cancer (RTOG 0617): a randomised, two-by-two factorial phase 3 study.Lancet Oncol.2015;16(2):187-99. doi: 10.1016/S1470-2045(14)71207-0.
- Mesia R, Henke M, Fortin A, et al. Chemoradiotherapy with or without panitumumab in patients with unresected, locally advanced squamous-cell carcinoma of the head and neck (CONCERT-1): a randomised, controlled, open-label phase 2 trial.Lancet Oncol.2015;16(2):208-220. doi: 10.1016/S1470-2045(14)71198-2.
- Giralt J, Trigo J, Nuyts S, et al. Panitumumab plus radiotherapy versus chemoradiotherapy in patients with unresected, locally advanced squamous-cell carcinoma of the head and neck (CONCERT-2): a randomised, controlled, open-label phase 2 trial.Lancet Oncol.2015;16(2):221-32. doi: 10.1016/S1470-2045(14)71200-8.
- Jiang Y, Mackley HB, Kimchi ET, et al. Phase I dose escalation study of capecitabine and erlotinib concurrent with radiation in locally advanced pancreatic cancer.Cancer Chemother Pharmacol.2014;74(1):205-210.
- Das P, Eng C, Rodriguez-Bigas MA, et al. Preoperative radiation therapy with concurrent capecitabine, bevacizumab, and erlotinib for rectal cancer: a phase 1 trial.Int J Radiat Oncol Biol Phys.2014;88(2):301-305.
- Iyer R, Chhatrala R, Shefter T, et al. Erlotinib and radiation therapy for elderly patients with esophageal cancer - clinical and correlative results from a prospective multicenter phase 2 trial.Oncology.2013;85(1):53-8. doi: 10.1159/000351617.
- Iyengar P, Kavanagh BD, Wardak Z, et al. Phase II trial of stereotactic body radiation therapy combined with erlotinib for patients with limited but progressive metastatic non-small-cell lung cancer.J Clin Oncol.2014;32(34):3824-3830. doi: 10.1200/JCO.2014.56.7412.
- Prados MD, Chang SM, Butowski N, et al. Phase II study of erlotinib plus temozolomide during and after radiation therapy in patients with newly diagnosed glioblastoma multiforme or gliosarcoma.J Clin Oncol.2009;27(4):579-584. doi: 10.1200/JCO.2008.18.9639.
- Martins RG, Parvathaneni U, Bauman JE, et al. Cisplatin and radiotherapy with or without erlotinib in locally advanced squamous cell carcinoma of the head and neck: a randomized phase II trial.J Clin Oncol.2013;31(11):1415-1421. doi: 10.1200/JCO.2012.46.3299.
- Corn PG, Song DY, Heath E, et al. Sunitinib plus androgen deprivation and radiation therapy for patients with localized high-risk prostate cancer: Results from a multiinstitutional phase 1 study.Int J Radiat Oncol Biol Phys.2013;86(3):540-545. doi: 10.1016/j.ijrobp.2012.12.029.
- Wuthrick EJ, Kamrava M, Curran WJ, Jr, et al. A phase 1b trial of the combination of the antiangiogenic agent sunitinib and radiation therapy for patients with primary and metastatic central nervous system malignancies.Cancer.2011;117(24):5548- 5559. doi: 10.1002/cncr.26216.
- Tong CC, Ko EC, Sung MW, et al. Phase II trial of concurrent sunitinib and imageguided radiotherapy for oligometastases.PLoS One.2012;7(6):e36979. doi: 10.1007/s00204-012-1002-4.
- Schenkein DP. Preclinical data with bortezomib in lung cancer.Clin Lung Cancer.2005;7(suppl 2):S49-S55.
- Zhao Y, Foster NR, Meyers JP, et al. A phase I/II study of bortezomib in combination with paclitaxel, carboplatin, and concurrent thoracic radiation therapy for non-small-cell lung cancer: North Central Cancer Treatment Group (NCCTG)-N0321.J Thorac Oncol.2015;10(1):172-180. doi: 10.1097/ JTO.0000000000000383.
- Pugh TJ, Chen C, Rabinovitch R, et al. Phase I trial of bortezomib and concurrent external beam radiation in patients with advanced solid malignancies.Int J Radiat Oncol Biol Phys.2010;78(2):521-526. doi: 10.1016/j.ijrobp.2009.07.1715.
- Kubicek GJ, Werner-Wasik M, Machtay M, et al. Phase I trial using proteasome inhibitor bortezomib and concurrent temozolomide and radiotherapy for central nervous system malignancies.Int J Radiat Oncol Biol Phys.2009;74(2):433-439.
- O’Neil BH, Raftery L, Calvo BF, et al. A phase I study of bortezomib in combination with standard 5-fluorouracil and external-beam radiation therapy for the treatment of locally advanced or metastatic rectal cancer.Clin Colorectal Cancer.2010;9(2):119-125. doi: 10.3816/CCC.2010.n.017.
- Argiris A, Duffy AG, Kummar S, et al. Early tumor progression associated with enhanced egfr signaling with bortezomib, cetuximab, and radiotherapy for head and neck cancer.Clin Cancer Res.2011;17(17):5755-5764. doi: 10.1158/1078-0432.CCR-11-0861.
- Lane AA, Chabner BA. Histone deacetylase inhibitors in cancer therapy.J Clin Oncol.2009;27(32):5459-5468. doi: 10.1200/JCO.2009.22.1291.
- Ree AH, Dueland S, Folkvord S, et al. Vorinostat, a histone deacetylase inhibitor, combined with pelvic palliative radiotherapy for gastrointestinal carcinoma: the pelvic radiation and vorinostat (PRAVO) phase 1 study.Lancet Oncol.2010;11(5):459-464. doi: 10.1016/S1470-2045(10)70058-9.
- Shi W, Lawrence YR, Choy H, et al. Vorinostat as a radiosensitizer for brain metastasis: a phase I clinical trial.J Neurooncol.2014;118(2):313-319. doi: 10.1007/s11060-014-1433-2.
- Manegold PC, Paringer C, Kulka U, et al. Antiangiogenic therapy with mammalian target of rapamycin inhibitor RAD001 (everolimus) increases radiosensitivity in solid cancer.Clin Cancer Res.2008;14(3):892-900. doi: 10.1158/1078-0432.CCR- 07-0955.
- Deutsch E, Le Pechoux C, Faivre L, et al. Phase I trial of everolimus in combination with thoracic radiotherapy in non-small cell lung cancer.Ann Oncol.2015;26(6):1223-1229. doi: 10.1093/annonc/mdv105.
- Ma DJ, Galanis E, Anderson SK, et al. A phase II trial of everolimus, temozolomide, and radiotherapy in patients with newly diagnosed glioblastoma: NCCTG N057K.Neuro Oncol.2015;17(9):1261-1269. doi: 10.1093/neuonc/nou328.
- Sarkaria JN, Galanis E, Wu W, et al. North Central Cancer Treatment Group phase I trial N057K of everolimus (RAD001) and temozolomide in combination with radiation therapy in patients with newly diagnosed glioblastoma multiforme.Int J Radiat Oncol Biol Phys.2011;81(2):468-475. doi: 10.1016/j.ijrobp.2010.05.064.
- Brunner TB, Geiger M, Grabenbauer GG, et al. Phase I trial of the human immunodeficiency virus protease inhibitor nelfinavir and chemoradiation for locally advanced pancreatic cancer.J Clin Oncol.2008;26(16):2699-2706. doi: 10.1200/JCO.2007.15.2355.
- Buijsen J, Lammering G, Jansen RL, et al. Phase I trial of the combination of the Akt inhibitor nelfinavir and chemoradiation for locally advanced rectal cancer.Radiother Oncol.2013;107(2):184-188. doi: 10.1016/j.radonc.2013.03.023.
- Butowski N, Chang SM, Lamborn KR, et al. Enzastaurin plus temozolomide with radiation therapy in glioblastoma multiforme: a phase I study.Neuro Oncol.2010;12(6):608-613. doi: 10.1093/neuonc/nop070.
- Int J Radiat Oncol Biol Phys. 2014;88(5):986-997. doi: 10.1016/j.ijrobp.2013.08.035.
- Rekers NH, Troost EG, Zegers CM, et al. Stereotactic ablative body radiotherapy combined with immunotherapy: present status and future perspectives.Cancer Radiother.2014;18(5-6):391-395. doi: 10.1016/j.canrad.2014.06.012.
- Postow MA, Callahan MK, Barker CA, et al. Immunologic correlates of the abscopal effect in a patient with melanoma.N Engl J Med.2012;366(10):925-931. doi: 10.1056/NEJMoa1112824.
- Shin SM, Chouake RJ, Sanfilippo NJ, et al. Feasibility and efficacy of local radiotherapy with concurrent novel agents in patients with multiple myeloma.Clin Lymphoma Myeloma Leuk.2014;14(6):480-484. doi: 10.1016/j.clml.2014.07.010.
- Katz DA, Abrams RA, Sclamberg JS, Usha L. Radiosensitizing effect of anti-HER2/ neu agents: report of 2 cases and review of the literature.Pract Radiat Oncol.2015;5(2):e61-65. doi: 10.1016/j.prro.2014.06.006.
- Lawrence YR, Glass C, Symon Z, et al. Phase I trials involving radiation therapy, quantifying the risks.J Med Imaging Radiat Oncol.2013;57(6):719-724. doi: 10.1111/1754-9485.12082.
- Colevas AD, Brown JM, Hahn S, et al, Radiation Modifier Working Group of the National Cancer Institute. Development of investigational radiation modifiers.J Natl Cancer Inst.2003;95(9):646-651.
- Harrington KJ, Billingham LJ, Brunner TB, et al. Guidelines for preclinical and early phase clinical assessment of novel radiosensitisers.Br J Cancer.2011;105(5):628-639. doi: 10.1038/bjc.2011.240.

Novel Approaches Focus on Limiting Toxicity in Older Patients With ALL
April 22nd 2024The major challenges for clinicians treating older patients with acute lymphoblastic leukemia surround the emergence of resistance to existing therapies and the toxicities associated with current chemotherapies.
Read More
Ornstein Advises on Starting Dose and Management of Lenvatinib in RCC
April 21st 2024During a Case-Based Roundtable® event, Moshe Ornstein, MD, MA, provided guidance on dosing and toxicity concerns in a patient treated with lenvatinib plus pembrolizumab for advanced renal cell carcinoma.
Read More
Rugo Surveys Peers on Treatment After Metastatic Relapse of HR+, HER2– Breast Cancer
April 20th 2024During a Case-Based Roundtable® event, Hope S. Rugo, MD, FASCO, moderated a discussion on treatment options for a patient whose breast cancer recurred several years after adjuvant therapy.
Read More