The Rapid Uptake of Immunotherapy in Bladder Cancer
Immunotherapy agents have been rapidly absorbed into the treatment paradigm for various cancer types. Nowhere is this more obvious than in the treatment for patients with bladder cancer. In May alone, 3 checkpoint inhibitors were approved for use in bladder cancer, bringing the total of checkpoint inhibitors approved in this field to 5 agents.
Immunotherapy agents have been rapidly absorbed into the treatment paradigm for various cancer types. Nowhere is this more obvious than in the treatment for patients with bladder cancer. In May alone, 3 checkpoint inhibitors were approved for use in bladder cancer, bringing the total of checkpoint inhibitors approved in this field to 5 agents.
Following such a surge in such a short time, it can be difficult to keep track of the new drugs, what sets them apart, and where we stand in treating patients with bladder cancer with immunotherapy.
HISTORY OF IMMUNOTHERAPY IN BLADDER CANCER
Prior to 2016, Bacillus Calmette-Guérin (BCG) was the only immunotherapy agent to show activity in patients with urothelial carcinoma, the most common type of bladder cancer. Originally created as a tuberculosis vaccine in the 1920s,1 BCG is a unique strain of mycobacterium bovis that activates the immune system and induces an in ammatory response. The drug was FDA-approved for use in bladder cancer in 1990, and it was the first signal that urothelial carcinoma is an immune-responsive tumor.2BCG remains a standard of care in patients with high-risk nonmuscle invasive bladder cancer.1,3
Following BCG, studies investigated the use of interferons as a potential therapy for patients with bladder cancer, particularly those with nonmuscle invasive disease.1Yet, they were unable to provide a benefit in these patients.
“Bladder cancer has, in fact, been one of the first diseases for which immunotherapy was active, and we need to go back [more than] 15 years ago [to] when BCG was shown to be effective,” Joaquim Bellmunt, MD, PhD, said in a presentation on immunotherapy options in bladder cancer. “Since then, nothing has happeneduntil quite recently when the checkpoint inhibitors came.”
BEGINNING OF THE CHECKPOINTS IN BLADDER CANCER
Atezolizumab (Tecentriq) was the first checkpoint inhibitor to be approved for use in bladder cancer (F I G U R E ). The PD-L1 inhibitor was approved by the FDA on May 18, 2016, for the treatment of patients with locally advanced or metastatic urothelial carcinoma (mUC) whose disease has progressed during or after platinum-based chemotherapy or within 12 months of having received platinum-containing chemotherapy, either before or after surgery. The accelerated approval was based on data from the phase II IMvigor 210 study, which investigated atezolizumab in patients with locally advanced or mUC. It had previously received a breakthrough therapy designation for patients with PD-L1positive metastatic bladder cancer in March 2016.
In the IMvigor 210 study, atezolizumab demonstrated an overall response rate (ORR) of 14.8% (95% CI, 11.1%-19.3%) among 310 patients.4By PD-L1 expression, atezolizumab showed an ORR of 26% (95% CI, 17.7%-35.7%) in patients with expression ≥5% on tumor-in ltrating immune cells and 9.5% (95% CI, 5.9%- 14.3%) in patients with expression below 5% (n = 210). The median duration of response (DOR) was not reached.
Ten patients discontinued treatment due to adverse events (AEs) and 3 patients died due to sepsis, pneumonitis, or intestinal obstruction. Grade 3/4 AEs included urinary tract infection (9%), anemia (8%), fatigue (6%), dyspnea (4%), and hematuria (3%).
Although the drug was approved regardless of PD-L1 expression, the PD-L1 assay Ventana PD-L1 (SP142) was approved at the same time as a complementary diagnostic.
On February 2, 2017, nivolumab (Opdivo) was granted an accelerated approval in the second line as well. The treatment was approved for use in patients with locally advanced unresectable or mUC following progression on a platinum-containing regimen based on ndings from the phase II CheckMate-275 trial; the PD-1 inhibitor had received a breakthrough therapy designation for this indication in June 2016. Nivolumab was also approved for patients who have disease progression within 12 months of neoadjuvant or adjuvant treatment with platinum-containing chemotherapy.5
The ORR in the CheckMate-275 trial was 19.6% of 270 patients with platinum-refractory metastatic urothelial carcinoma with complete responses (CRs) in 3%.6 The median progression-free survival (PFS) was 2.0 months, and the median overall survival (OS) was 8.7 months. The median DOR was not reached in this trial either.
Among patients with PD-L1 expression on ≥1% of cells, the median PFS was 3.55 months and the OS was 11.3 months compared with 1.87 months and 5.95 months in those with no PD-L1 expression, respectively.
The most common AEs during the trial were fatigue (16.7%), pruritus (9.3%), diarrhea (8.9%), decreased appetite (8.1%), hypothyroidism (7.8%), nausea (7.0%), asthenia (5.9%), rash (5.9%), and pyrexia (5.6%). Treatment-related AEs were experienced by 64.4% of patients; 17.8% were grade 3/4. Quality of life was also improved from baseline, according to Global Health Status Scale measurements.
The FDA recommended a at dose of 240-mg nivolumab intra- venously in urothelial carcinoma every 2 weeks.5
Atezolizumab was granted an additional indication by the FDA on April 17, 2017, for the frontline treatment of cisplatin-ineligible patients with locally advanced or mUC based on a cohort of cisplatin-ineligible, treatment-naïve patients from the same IMvigor 210 trial. This was the rst checkpoint inhibitor to be granted an approval in the rst-line setting in bladder cancer.
The ORR was 23.5% (95% CI, 16.2%-32.2%) from the 119 eligible patients in the cohort, with a CR rate of 6.7%.7 The median PFS was 2.7 months (95% CI, 2.1-4.2), and the median OS was 15.9 months (95% CI, 10.4 to not estimable).
Patients with PD-L1 expression ≥5% (n = 32) had an ORR of 28.1% (95% CI, 13.8%-46.8%), with a CR rate of 6.3%. In those with PD-L1 expression below 5%, the ORR was 21.8% (95% CI, 13.7%-32.0%) and the CR rate was 6.9%. Additionally, an association between tumor mutation load and response was noted.
In this cohort, 5 patients discontinued atezolizumab therapy and 5 patients died, due to sepsis, cardiac arrest, myocardial infarction, respiratory failure, and respiratory distress. An additional patient had in ammation of the brain due to the herpes simplex virus and disease progression. Similar common grade 3/4 AEs were experienced in this cohort, including fatigue (8%), urinary tract infection (5%), anemia (7%). Additional grade 3/4 AEs included diarrhea (5%), increase in the level of creatinine in the blood (5%), increase of the liver enzyme alanine transaminase (4%), hyponatre- mia (15%), decreased appetite (3%), and back/neck pain (3%).
Both approvals for atezolizumab were contingent upon results from the phase III con rmatory study, IMvigor 211.
A BUSY MONTH IN BLADDER CANCER
On May 1, 2017, durvalumab (Imfinzi), another PD-L1 inhibitor, was granted an accelerated approval by the FDA in the second-line setting for the treatment of patients with locally advanced or mUC who have disease progression during or following platinum-containing chemotherapy or who have disease progression within 12 months of neoadjuvant or adjuvant treatment with platinum-containing chemotherapy. The approval is contingent upon results from a confirmatory trial.
A breakthrough therapy designation had been granted to the agent in February 2016 for patients with PD-L1positive inoperable or mUC following progression on prior treatment with a platinum-based regimen.
The FDA granted the approval based on the results of a single-arm trial of 182 patients with locally advanced or mUC who experienced disease progression following platinum-containing chemotherapy. The ORR among the patients was 17% (95% CI, 11.9%-23.3%) by blinded independent central review.8In patients with high PD-L1 expression (n = 95), as assessed by the Ventana PD-L1 (SP263) assaythe complementary diagnostic approved for use with durvalumab—the ORR was 26.3% (95% CI, 17.8%-36.4%) compared with 4.1% (95% CI, 0.9%-11.5%) among patients with low or no PD-L1 expression (n = 73). Thirty-one patients achieved a response, including 5 CRs and 26 partial responses (PRs).
Common all-grade AEs included fatigue, musculoskeletal pain, constipation, decreased appetite, nausea, peripheral edema, and urinary tract infection. Immune-related AEs included pneumonitis, hepatitis, colitis, thyroid disease, adrenal insuf ciency, and diabetes.
Next, the FDA granted an accelerated approval to avelumab (Bavencio) on May 9 for patients with locally advanced or mUC with disease progression during or following platinum-containing chemotherapy or within 12 months of neoadjuvant or adju- vant platinum-containing chemotherapy.
The approval was based on data from a cohort of 242 patients with locally advanced or mUC from the single-arm, open-label, phase Ib JAVELIN Solid Tumor trial. Patients who had been fol- lowed for at least 13 weeks (n = 226) had an ORR of 13.3% (95% CI, 9.1%-18.4%), and those who had been followed for at least 6 months (n = 161) had an ORR of 16.1% (95% CI, 10.8%-22.8%).9 The median time to response was 2.0 months (range, 1.3-11.0) and the median DOR was not reached in either group.
Among patients followed for 6 months or more, there were 9 CRs (5.6%) and 17 PRs (10.6%) versus 9 CRs (4%) and 21 PRs (9.3%) among those followed for at least 13 weeks. Response rates did not vary signi cantly according to PD-L1 expression levels.
All-grade AEs frequently reported included fatigue (41%), infusion-related reaction (30%), musculoskeletal pain (25%), nausea (24%), decreased appetite (21%), and urinary tract infection (21%). Serious AEs occurred in 41% of patients and included cases of urinary tract infection/urosepsis, abdominal pain, musculoskeletal pain, increased creatinine levels/renal failure, dehydration, hematuria/urinary tract hemorrhage, intestinal obstruction/small intestine obstruction, and pyrexia. Deaths due to AEs occurred in 14 patients (6%), and these were due to pneumonitis, respiratory failure, sepsis/urosepsis, cerebrovascular accident, or gastrointestinal issues.
The FDA noted that the recommended dose for avelumab is 10 mg/kg intravenously over 60 minutes every 2 weeks, and indicated that the accelerated approval is contingent upon results of a confirmatory trial.
Disrupting the long stream of good news in the field, Genentech, the manufacturer of atezolizumab, announced on May 9 that the drug had failed to meet its primary endpoint in the second-line setting in the con rmatory phase III IMvigor 211 trial, on which the rst approval of atezolizumab in bladder cancer was based.10The trial was unable to show an improvement in OS with atezolizumab therapy compared with chemotherapy for patients with previously treated locally advanced or mUC. Genentech noted that full results from the IMvigor 211 trial will be presented later this year after the data have been further examined.
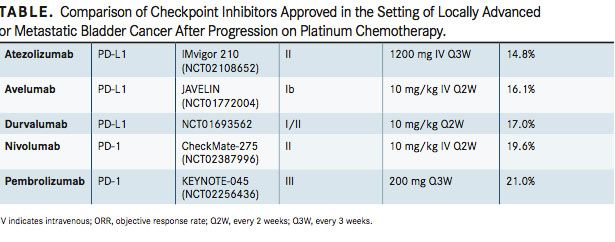
Most recently, on May 18, pembrolizumab (Keytruda) received FDA approval for use in bladder cancer. The PD-1 inhibitor was approved for the treatment of patients with locally advanced or mUC who have disease progression during or following platinum-containing chemotherapy or within 12 months of neoadjuvant or adjuvant treatment with platinum-containing chemotherapy. A second accelerated approval was awarded to frontline pembrolizumab for patients with locally advanced or mUC who are ineligible for cisplatin-containing chemotherapy, although this approval is contingent upon results from a con rmatory trial.11
The second-line approval was based on ndings from the phase III KEYNOTE-045 trial that demonstrated a 27% reduction in the risk of death compared with chemotherapy in patients with advanced urothelial carcinoma who had progressed following prior treatment and a 43% reduction in the risk of death over chemotherapy in patients with a combined positive score (CPS) of PD-L1 expression ≥10% (HR, 0.57; 95% CI, 0.37-0.88; P = .0048).12Patients on the trial had locally advanced or metastatic, unresectable urothelial carcinoma of the renal pelvis, ureter, bladder, or urethra, and had progressed after 1 to 2 lines of platinum-based chemotherapy or had experienced recurrence after 12 months of chemotherapy. They were treated with either pembrolizumab or chemotherapy options of paclitaxel, docetaxel, or vin unine.
The median OS with pembrolizumab was 10.3 months (95% CI, 8.0-11.8) compared with 7.4 months (95% CI, 6.1-8.3) in patients treated with chemotherapy (HR, 0.73; 95% CI, 0.59-0.91; P = .004). In patients with a CPS ≥10%, calculated with both tumor cell and infiltrating immune cell expression of PD-L1 by PD-L1 IHC 22C3 pharmDx assay measurement, the median OS was 8.0 months (95% CI, 5.0-12.3) with pembrolizumab compared with 5.2 months (95% CI, 4.0-7.4) in those treated with chemotherapy.
The overall median PFS was 2.1 months with pembrolizumab, which was not found to be superior to the median PFS of 3.3 months experienced with chemotherapy (P = .833).
The ORR was 21.0% with immunotherapy compared with 11.0% with chemotherapy (P = .002), and the CR rates were 7.0% and 3.3%, respectively.
The rates of all-grade (60.9% vs 90.2%) and grade 3-5 (15.0% vs 49.4%) treatment-related AEs were lower with pembrolizumab compared with chemotherapy, respectively. Common treat- ment-related AEs with pembrolizumab included fatigue (13.9% vs 27.8% with chemotherapy), nausea (10.9% vs 24.3%), diarrhea (9.0% vs 12.9%), asthenia (5.6% vs 14.1%), and anemia (3.4% vs 24.7%). However, the rate of pruritus was higher in the pembrolizumab arm than the chemotherapy arm (19.5% vs 2.7%).
Immune-related AEs that were higher with pembrolizumab compared with chemotherapy, respectively, included thyroid abnormalities (9.4% vs 1.6%), pneumonitis (4.1% vs 0.4%), and colitis (2.3% vs 0.4%). Treatment-related AEs leading to discon- tinuation of treatment occurred in 15 patients in the pembroli- zumab arm and in 28 patients in the chemotherapy group. Each arm had 4 treatment-related deaths.
For the frontline approval, data came from the phase II KEYNOTE-052 trial with 370 cisplatin-ineligible patients with locally advanced or mUC. The ORR was 28.6% (95% CI, 24%-34%) after a median follow-up of 7.8 months, and the median DOR was not reached.11CRs were noted in 7% and PRs in 22% of patients.
Five patients discontinued pembrolizumab due to a treatment-related AE; no deaths were attributed to a treatment-related AE.13All-grade treatment-related AEs were experienced in 67% of patients, including fatigue (14%), pruritus (12%), pyrexia (8%), decreased appetite (7%), diarrhea (7%), rash (7%), chills (6%), hypothyroidism (6%), and nausea (6%). Grade 3/4 treatment-related AEs were experienced by 16% of patients and included fatigue (4%), muscle spasms (2%), decreased appetite (1%), and diarrhea (1%).
DETERMINING THE IMPACT OF RAPID APPROVALS
Following each of these approvals, the question has become: What does this mean for clinical practice now that there are so many available options? And, which agent should be used in which patients, and why?
“The trouble is that there is no comparative data, there’s no sequential data, so nobody knows what should be given first, and what should be given second. The response rates appear to be similar, the survival rates appear to be similar as well,” Daniel Petrylak, MD, said in an interview with Targeted Therapies in Oncology.
Many are also wondering how the news of atezolizumab failing to meet its primary endpoint in the second-line setting will affect its approval status, especially since the approval was contingent upon the results of this trial.
Experts are looking at ndings on these drugs comparatively to see which 1 should be the go-to regimen in each setting (TABLE). “The only thing that separates these drugs is how frequently you give them. Nivolumab is given every 2 weeks [and] atezolizumab is given every 3 weeks, so that’s an advantage, because you don’t have to come in as often,” noted Petrylak, professor of medicine (medical oncology) and professor of urology at Yale School of Medicine and co-director of the Signal Transduction Research Program at Yale Cancer Center, as an example of a factor that may separate 1 drug from the rest. Beyond simple comparisons across trials, specific sequencing preferences will depend upon the results of randomized trials.
In the meantime, the National Comprehensive Cancer Network guidelines, which were updated on May 25 to incorporate these most recent changes in the eld, includes all 5 of these checkpoint inhibitors as options for subsequent systemic therapy in patients with locally advanced or metastatic disease.3Yet, they gave pem- brolizumab alone a category 1 recommendation in this setting as it was the only agent to have data from a phase III trial.
A number of ongoing trials are continuing to look at these agents, including in combination regimens. Perhaps those results will impact how these agents fall into place in the treatment paradigm for bladder cancer. Trials are also investigating these checkpoint inhibitors in additional settings, including as maintenance, adjuvant, and neoadjuvant therapies.14
Results from the IMvigor 130 study (NCT02807636) are eagerly anticipated, for example, to support the approval for frontline atezolizumab in cisplatin-ineligible patients with locally advanced or mUC.
The phase III DANUBE trial (NCT02516241) is also of interest to determine the ef cacy of durvalumab in combination with tremelimumab, an antiCTLA-4 antibody, in patients with locally advanced or mUC. Nivolumab is also being investigated with a CTLA-4 checkpoint inhibitor, ipilimumab (Yervoy) in patients with mUC in the ongoing phase I/II CheckMate-032 trial (NCT01928394).
“My belief is that in metastatic urothelial cancer, immunotherapy will be a standard of care at some point in the patient’s course of therapy, whether it be frontline or second line, unless there are clear contrary indications for the patient to receive it,” Arjun Balar, MD, assistant professor of medicine and director, Genitourinary Medical Oncology Program, NYU Langone Medical Center, said in an interview. “I believe the scope of [these therapies] will be very broad and essentially applied to almost all patients with advanced bladder cancer at some point in their disease.”
References:
- Donin NM, Lenis AT, Holden S, et al. Immunotherapy for the treatment of urothelial carcinoma. J Urol. 2017;197(1):14-22. doi: 10.1016/j.juro.2016.02.3005.
- Davarpanah NN, Yuno A, Trepel JB, Apolo AB. Immunotherapy: a new treatment paradigm in blad- der cancer. Curr Opin Oncol. 2017;29(3):184-195. doi: 10.1097/CCO.0000000000000366.
- National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology: Bladder Cancer. V5.2017. NCCN website. https://www.nccn.org/professionals/physician_gls/pdf/blad- der.pdf. Published May 25, 2017. Accessed June 1, 2017.
- Rosenberg JE, Ho man-Censits J, Powles T, et al. Atezolizumab in patients with locally advanced and metastatic urothelial carcinoma who have progressed following treatment with platinum-based chemotherapy: a single-arm, multicentre, phase 2 trial. Lancet. 2016;387(10031):1909-1920. doi: 10.1016/S0140-6736(16)00561-4.
- Opdivo (nivolumab) [prescribing information]. Princeton, NJ: Bristol-Myers Squibb Company; 2017. https:// www.accessdata.fda.gov/drugsatfda_docs/label/2017/125554s031lbl.pdf. Accessed June 1, 2017.
- Galsky MD, Retz M, Siefker-Radtke AO, et al. E cacy and safety of nivolumab monotherapy in patients with metastatic urothelial cancer (mUC) who have received prior treatment: Results from the phase II CheckMate-275 study. Presented at: 2016 ESMO Congress; October 7-11, 2016; Copen- hagen, Denmark. Abstract LBA31_PR.
- Balar AV, Galsky MD, Rosenberg JE, et al; IMvigor 210 Study Group. Atezolizumab as rst-line treatment in cisplatin-ineligible patients with locally advanced and metastatic urothelial carcinoma: a single-arm, multicentre, phase 2 trial. Lancet. 2017;389(10064):67-76. doi: 10.1016/S0140- 6736(16)32455-2.
- Durvalumab (Im nzi). FDA website. https://www.fda.gov/drugs/informationondrugs/approved- drugs/ucm555930.htm. Updated May 1, 2017. Accessed June 1, 2017.
- FDA grants accelerated approval to avelumab for urothelial carcinoma. FDA website. https:// www.fda.gov/drugs/informationondrugs/approveddrugs/ucm557162.htm. Updated May 9, 2017. Accessed June 1, 2017.
- Genentech provides update on phase III study of Tecentriq (atezolizumab) in people with previ- ously treated advanced bladder cancer [press release]. South San Francisco, CA: Genentech; May 9, 2017. https://www.gene.com/media/press-releases/14665/2017-05-09/genentech-provides- update-on-phase-iii-s. Accessed June 1, 2017.
- Pembrolizumab (Keytruda): advanced or metastatic urothelial carcinoma. FDA website. https:// www.fda.gov/Drugs/InformationOnDrugs/ApprovedDrugs/ucm559300.htm. Updated May 19, 2017. Accessed June 1, 2017.
- Bellmunt J, de Wit R, Vaughn DJ, et al. Keynote-045: open-label, phase III study of pembrolizumab ver- sus investigator’s choice of paclitaxel, docetaxel, or vin unine for previously treated advanced urothelial cancer. Presented at: 2016 SITC Annual Meeting; November 9-13, 2016; National Harbor, MD.
- Balar A, Bellmunt J, O’Donnell PH, et al. Pembrolizumab (pembro) as rst-line therapy for advanced/ unresectable or metastatic urothelial cancer: Preliminary results from the phase II KEYNOTE-052 study. Presented at: 2016 ESMO Congress; October 7-11, 2016; Copenhagen, Denmark. Abstract LBA32.
- Farina MS, Lundgren KT, Bellmunt J. Immunotherapy in urothelial cancer: recent results and fu- ture perspectives [published online May 11, 2017]. Drugs. 2017. doi: 10.1007/s40265-017-0748-7.
FDA Approves Nogapendekin Alfa Inbakicept for BCG-Unresponsive NMIBC Carcinoma In Situ
April 22nd 2024Patients with Bacillus Calmette-Guérin-unresponsive non-muscle-invasive bladder cancer carcinoma in situ now have a new treatment option following the FDA’s approval of nogapendekin alfa.
Read More
Novel Approaches Focus on Limiting Toxicity in Older Patients With ALL
April 22nd 2024The major challenges for clinicians treating older patients with acute lymphoblastic leukemia surround the emergence of resistance to existing therapies and the toxicities associated with current chemotherapies.
Read More
Ornstein Advises on Starting Dose and Management of Lenvatinib in RCC
April 21st 2024During a Case-Based Roundtable® event, Moshe Ornstein, MD, MA, provided guidance on dosing and toxicity concerns in a patient treated with lenvatinib plus pembrolizumab for advanced renal cell carcinoma.
Read More
Rugo Surveys Peers on Treatment After Metastatic Relapse of HR+, HER2– Breast Cancer
April 20th 2024During a Case-Based Roundtable® event, Hope S. Rugo, MD, FASCO, moderated a discussion on treatment options for a patient whose breast cancer recurred several years after adjuvant therapy.
Read More
