Tumor Sidedness Findings Could Change Trials and Treatments in Colorectal Cancer
The impact of the recent findings regarding tumor sidedness in patients with metastatic colorectal cancer are potentially practice-changing, with data demonstrating that patients with right-sided tumors have a poorer prognosis than those with left-sided tumors. In addition, sidedness could be clinically relevant as a predictive biomarker of response to standard frontline treatments.
Alan P. Venook, MD
The impact of the recent findings regarding tumor sidedness in patients with metastatic colorectal cancer (mCRC) are potentially practice-changing, with data demonstrating that patients with right-sided tumors have a poorer prognosis than those with left-sided tumors. In addition, sidedness could be clinically relevant as a predictive biomarker of response to standard frontline treatments.
Yet these findings are still immature. Further studies are required to confirm and to explore the molecular and biological reasons behind the differences between left- and right-sided tumors, before the findings can be integrated into clinical practice.
CALGB/SWOG 80405 JUMPSTARTS FOCUS ON SIDEDNESS
Although the importance of tumor sidedness in colorectal cancer has been referenced since 2001, the importance of this issue was pushed to the forefront in June during the 2016 ASCO Annual Meeting.
During the ASCO meeting, Alan P. Venook, MD, presented a retrospective subgroup analysis from the CALGB/SWOG 80405 trial that showed that patients with left-sided tumors had significantly longer median overall survival (OS) rates than patients with right-sided tumors.1Patients with right-sided tumors, consisting of tumors in the appendix, cecum, ascending colon, and hepatix flexure, had a median OS of 19.4 months (95% CI, 16.7-23.6) versus 33.3 months (95% CI, 31.4-35.7) in patients with left-sided tumors, consisting of tumors in the splenic exure, descending colon, sigmoid colon, and rectum (HR, 1.60; 95% CI, 1.37-1.86; P <.001). The results were similar when the transverse colon was allotted to the right side, as in other similar studies, and in patients with KRAS-mutant tumors.
In the study, 1137 treatment-naïve patients with KRAS wild-type (codons 12 and 13) mCRC were treated with FOLFIRI (irinotecan/5- fluorouracil [5-FU]/leucovorin) or mFOLFOX6 (oxaliplatin/5-FU/ leucovorin) with either bevacizumab (Avastin) or cetuximab (Erbitux). Patients with right-sided tumors treated with cetuximab had a median OS of 16.7 months (95% CI, 13.1-19.4) versus 36 months (95% CI, 32.6-40.3) in the left-sided tumors (HR, 1.98; 95% CI, 1.60- 2.46; P <.001). With bevacizumab therapy, patients with right-sided tumors had a median OS of 24.2 months (95% CI, 17.9-30.3) versus 31.4 months (95% CI, 28.3-33.6) for those with left-sided tumors (HR, 1.32; 95% CI, 1.05-1.65).
The analysis suggested that treatment with the anti-EGFR monoclonal antibody cetuximab was more beneficial for patients with left-sided tumors and bevacizumab improved outcomes for patients with right-sided tumors, which could potentially affect the frontline standard of care for patients with mCRC.
“What we saw, and what others have now replicated, is that patients with tumors on the right side of the colon had no benefit from cetuximab,” said Venook, the Shorenstein associate director for program development at the Helen Diller Family Comprehensive Cancer Center, and professor of medicine at the University of California, San Francisco, in an interview withTargeted Therapies in OncologyTM.
However, Venook, noted that this was a retrospective, unplanned analysis, so it cannot yet be confirmed that either drug is superior in the frontline based on tumor sidedness. However, similar results were seen in the FIRE-3 and CRYSTAL studies.
PROGNOSTIC/PREDICTIVE BIOMARKER?
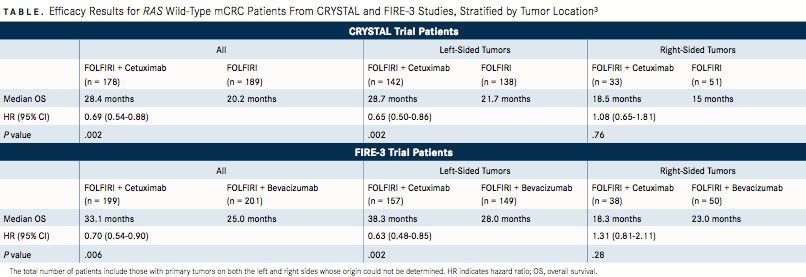
In the FIRE-3 trial, patients with KRAS (exon 2) codon 12 or 13 wild-type mCRC were treated with FOLFIRI plus cetuximab or FOLFIRI plus bevacizumab. The median OS for patients receiving FOLFIRI plus cetuximab was 33 versus 25 months in the bevaci- zumab arm.2When analyzed by primary tumor location, those with left-sided tumors (n = 306) showed a median OS of 38.3 versus 18.3 months in patients with right-sided tumors (n = 88) when treated with cetuximab (P <.0001). Patients with left-sided tumors treated with bevacizumab had a median OS of 28.0 versus 23.0 months in patients with right-sided tumors (P = .038).1
A recent retrospective analysis of FIRE-3 and CRYSTALanother study of anti-EGFR therapy for patients with mCRC—explored the predictive and prognostic nature of primary tumor location in patients with RAS wild-type tumors. The analysis demonstrated that patients with left-sided tumors had superior OS rates (TABLE) as well as superior progression-free survival and objective response rates compared with patients with right-sided tumors across both studies.3In addition, patients with left-sided tumors gained more of a benefit from treatment with cetuximab than patients with right-sided tumors did; meanwhile, patients with right-sided tumors did not benefit as much from treatment with standard therapy.
Consequently, sidedness could be both predictive of response to treatment with anti-EGFR therapy or standard therapy, and prog- nostic of patients' improved survival rates when harboring tumors in the left side of the colon. Venook similarly suggested the predic- tive and prognostic value of tumor sidedness in his analysis of the CALGB/SWOG 80405 trial.1
Sabine Tejpar, MD, and her co-authors also noted a significant interaction in multivariable models between tumor sidedness and treatment with regard to OS rates in both studies when also stratifying for sex, prior adjuvant therapy, and BRAF mutational status.3Tejpar et al recommended that primary tumor location be included as a stratification parameter in future trials of patients with mCRC, especially for trials including anti-EGFR therapies.
The prognostic nature of tumor sidedness may depend on the stage of the disease. A study of patients with CRC from the Surveillance Epidemiology and End Results Program database, which was presented at the 2016 ASCO Annual Meeting by Deborah Schrag, MD, MPH, survival rates favoring left-sided tumors were confirmed in patients with stage III or IV disease. In stage III, patients with left-sided tumors had a 71% rate of survival at 3 years versus 62% for those with right-sided tumors (unadjusted HR, 1.39; 95% CI, 1.32-1.46). In stage IV, the 3-year survival probability was 27% with left-sided tumors and 16% for those with right-sided tumors (unadjusted HR, 1.40; 95% CI, 1.35-1.46). However, the association between primary tumor location and prognosis was weaker or nonexistent in patients with stage I or II disease.4
POTENTIAL MOLECULAR AND BIOLOGICAL CAUSES
Further study is required to identify the molecular and biological causes that account for the differences in prognosis between left- and right-sided tumors.
“Hopefully, we will soon have the data to explain what is going on. It is not a function of right versus left side; it’s molecular features that appear to be nonrandomly distributed across the colon,” Venook commented. “I am hoping that we will have the opportunity to explain not only why patients do better on the left versus right with certain drugs, but also to prospectively understand why that is and how we should be treating patients.”
In the analysis of the CALGB/SWOG 80405 trial, Venook suggested that BRAF mutation status, microsatellite instability (MSI), and/or methylation could provide potential explanations.1
During the 2016 ASCO meeting, Schrag also suggested that there could be an association between sidedness and recently defined consensus molecular subtypes of CRC from an international consortium of experts.4The consortium differentiated the biological underpinnings of 4 subtypes: CMS1, tumors that are MSI-immune, CMS2 are canonical tumors, CMS3 are metabolic tumors, and CMS4 are mesenchymal tumors.5Schrag noted that patients with right-sided tumors were more common in the CMS1 and CMS3 subtypes, which are associated with BRAF and KRAS mutations, respectively. Both subtypes were associated with worse prognoses.4
“What we are hoping we will see is that there are molecular subtypes of CRC. We think there are 4, and some of them are overrepre- sented on the right or left side. We think that could explain much of the biological difference between right and left,” Venook said.
A prospective analysis of common mutations in patients with CRC noted that BRAF V600E and PIK3CA mutations were more prevalent in patients with RAS wild-type CRC tumors in the right side of the colon (P = .0001).6 BRAF V600E mutations occurred in 31.7% of right-sided tumors compared with BRAF non-V600E mutations in 8.1%, and PIK3CA occurred in 19.2% of right-sided tumors. “The existence of these mutations may affect anti-EGFR efficacy between right- and left-sided colorectal cancers,” the authors concluded in their report.
Disparities based on gender could also affect differences based on tumor location. Women over 65 years who develop CRC typically have a lower 5-year OS rate than men. This could be due to the fact that women have a higher risk of developing tumors in the right side of the colon. A study investigated this role further to illuminate other differences associated with gender and tumor location.7
Kim et al found that a number of hormonal and molecular factors may explain the larger percentage of right-sided tumors in women than in men (FIGURE). The lack of estrogen in older women could potentially relate to the increased risk of developing MSI-high CRC, which is more common in women and in right-sided tumors. Chromosomal instability, on the other hand, was more likely to be associated with left-sided tumors. Right-sided tumors show a link as well with the CpG island methylator phenotype and BRAF mu- tations. Hereditary non-polyposis colorectal cancer was also more likely to lead to right-sided tumors, while familial adenomatous pol- yposis led to left-sided tumors.
Perhaps including these and other features in future analyses of the effect of sidedness on treatment, or as a strati cation component in randomized clinical trials, could allow researchers to confirm the true underpinnings of tumor sidedness’ effect on outcomes.
Further molecular profiling will be essential to de ne the associations that primary tumor location has with gender, mutations, and other risk parameters. Defining these features will help to clarify tumor sidedness as a biomarker, as well as to personalize the treat- ment of patients with mCRC based on their characteristics.
References:
- Venook AP, Niedzwiecki D, Innocenti F, et al. Impact of primary (1o) tumor location on overall survival (OS) and progression-free survival (PFS) in patients (pts) with metastatic colorectal cancer (mCRC): analy- sis of CALGB/SWOG 80405 (Alliance). J Clin Oncol. 2016;34(suppl; abstr 3504).
- Heinemann V, Fischer von Weikersthal L, Decker T, et al. FOLFIRI plus cetuximab versus FOLFIRI plus bevacizumab as rst-line treatment for patients with metastatic colorectal cancer (FIRE-3): a randomised, open-label, phase III trial. Lancet Oncol. 2014;15(10):1065-1075. doi: 10.1016/S1470-2045(14)70330-4.
- Tejpar S, Stintzing S, Ciardiello F, et al. Prognostic and predictive relevance of primary tumor location in patients with RAS wild-type metastatic colorectal cancer: retrospective analyses of the CRYSTAL and FIRE-3 trials. JAMA Oncol. 2017;3(2):194-201. doi: 10.1001/jamaoncol.2016.3797.
- Schrag D, Weng S, Brooks G, et al. The relationship between primary tumor sidedness and prognosis in colorectal cancer. J Clin Oncol. 2016;34(suppl; abstr 3505).
- Guinney J, Dienstmann R, Wang X, et al. The consensus molecular subtypes of colorectal cancer. Nat Med. 2015;21(11):1350-1356. doi: 10.1038/nm.3967.
- Taniguchi H, Uehara K, Nakayama H, et al. The location of colorectal cancer (right- vs. left-sided colon and rectum) a ects the prevalence of BRAF V600E, non-V600E and PIK3CA mutations: a prospective registration study in the Aichi Cancer Network. Ann Oncol. 2016;27(suppl 6): 569P. doi: 10.1093/annonc/mdw370.117.
- Kim S-E, Paik HY, Yoon H, Lee JE, Kim N, Sung MK. Sex- and gender-speci c disparities in colorectal cancer risk. World J Gastroenterol. 2015;71(17):5167-5175. doi: 10.3748/wjg.v21.i17.5167.

Creating Solutions for a 'Continual State of Transition' in Cancer Care
April 15th 2024In a Peers & Perspectives in Oncology feature article, we focus on the importance of the transition-of-care process for patients with cancer as they move from the inpatient to outpatient setting, as well as between lines of therapy with comments from Marc J. Braunstein, MD, PhD, and Michael Shusterman, MD.
Read More