Evolving Paradigms in Immuno-Oncology: A New Paradigm for Evaluation of Tumor Response and Adverse Events
This feature covers the "A New Paradigm for Evaluation of Tumor Response and Adverse Events" section of the current Evolving Paradigms in Immuno-Oncology issue.
A New Paradigm for Evaluation of Tumor Response and Evaluation of Adverse Events
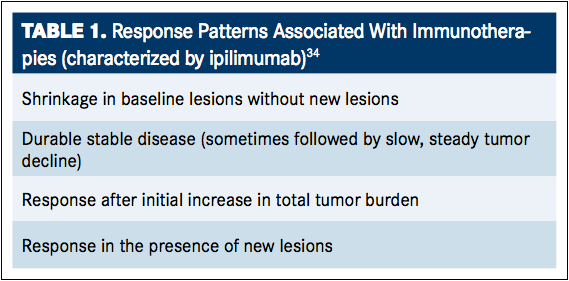
Checkpoint inhibition has demonstrated positive results in the treatment of various cancers, enhancing the immune system’s natural ability to identify and eliminate cancer cells, stopping tumor growth, and preventing cancer in a self-sustaining manner. However, the antitumor effects induced by immunotherapeutic agents require a cancer-specific immune response or the modification of the native immune process, which may take longer to mount compared with responses to chemotherapies or targeted agents. During the time from administration of immunotherapy treatment to the time of response, tumor growth and the presence of new lesions may occur. Because of this, the Response Evaluation Criteria in Solid Tumors (RECIST) and World Health Organization (WHO) criteria, designed to detect early effects of cytotoxic agents, may be inadequate in assessing the effects of immunotherapeutic agents. This became apparent during the treatment of melanoma with ipilimumab, where increased survival has been associated with four response patterns (TABLE 1).34It is important to note that although all four of these responses are associated with increased survival, response after initial increase in tumor burden or in the presence of new lesions could easily be mistaken for disease progression under standard response criteria. It is for this reason that immune-related response criteria (irRC) have been developed as a tool for the evaluation of antitumor response beyond those described by RECIST and for the continuation of treatment with immunotherapeutic agents in instances of seemingly ambiguous response.34,35Although these response patterns were observed in patients with melanoma treated with ipilimumab, the same patterns are likely to be observed in patients using any of the available checkpoint inhibitors.
A wide spectrum of immune-related adverse events (irAEs) has also been identified, including enterocolitis, hepatitis, dermatitis, and endocrinopathies. irAEs are more common with CTLA-4 blockade than with antiPD-1/PD-L1 therapies and anti–PD-1/PD-L1 therapies, are associated with a slightly different toxicity profiles than anti–CTLA-4 therapies. In particular, anti-PD-1/PD-L1 therapies are associated with different organ-specific inflammatory profiles, such as pneumonitis instead of colitis, which is more prominent with CTLA-4 blockade. However, general AEs associated with anti–PD-1/PD-L1 therapy still exist, and include fatigue (the most common irAE), pyrexia, chills, and infusion reactions. Skin rash is the most common organ-specific irAE associated with anti–PD-1/PD-L1 therapy. The most serious irAEs associated with anti–PD-1 therapy include pneumonitis, colitis, hepatitis, thyroid dysfunction, nephritis, and renal dysfunction.36
Biomarkers and Checkpoint Inhibition
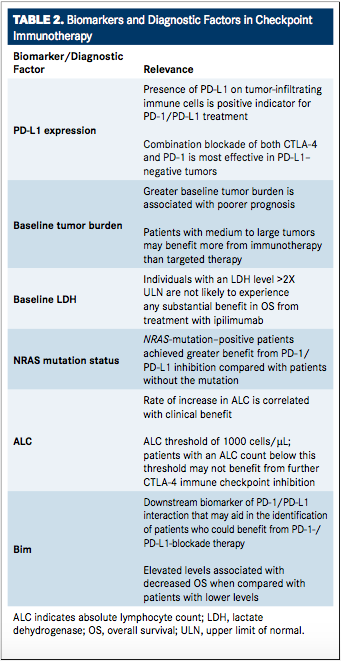
Optimal treatment strategies for individual patients can also be identified based on predictors of patient response, such as genetic analysis, biomarker identification, and other diagnostic indicators of immunologic reaction (TABLE 2). Recent studies with antiCTLA-4 and anti–PD-1 therapies alone, as well as in combination, have revealed that the use of PD-L1 as a biomarker may allow clinicians to make more informed decisions regarding the optimal use of PD-1/ PD-L1 therapies and aid in risk-benefit analyses when considering combination checkpoint therapy versus monotherapy.37,38A downstream biomarker of PD-1/PD-L1 interaction, Bim, has also been identified to be associated with decreased OS in patients with cancer and may aid in the identification of patients who could benefit from PD-1/PD-L1 blockade therapy.39The expression of PD-L1 on tumorinfiltrating immune cells (demonstrated by anti-PD-L1 antibody immunohistochemistry [IHC] optimized for staining of formalin-fixed, paraffin-embedded tissue samples) is currently recognized as a positive indicator for use of antiPD-1 and anti–PD-L1 treatment.32,38,40AntiPD-L1 treatment is most successful in patients with pre-existing immunity suppression by PD-1/PD-L1 pathway (indicated directly by PD-L1 expression), allowing for reinvigoration of the immune system with treatment. Similarly, a heightened level of CTLA-4 prior to treatment has also been correlated with increased response to anti–PD-L1 therapy.32A combination blockade of both CTLA-4 and PD-1 has demonstrated the greatest benefit despite the possibility for additional AEs in patients with PD-L1−negative tumors compared with monotherapy with either agent alone. This combination also demonstrated efficacy in patients with PD-L1positive tumors, although considerations for risk versus benefit must be weighed.37
Whole-exome sequencing, via next-generation sequencing (NGS), may provide another tool for treatment selection. By processing and sequencing the complete set of mutation-harboring genes within a tumor not normally expressed in healthy cells (ie, total mutation load), greater accuracy in specific immunotherapy selection for individual patients may be determined.41In a recent study, whole-exome sequencing data from a large set of tumors (n = 1098) was analyzed and a predicted total mutation load (PTML) was developed based on a small number of genes (n = 170) that allows for NGS panels to easily screen patients and identify those who may benefit most from checkpoint blockade therapies based on the presence or absence of these specific mutations.41This algorithm was then tested retrospectively in data sets from patients with cancer (including patients who had received immunotherapy) and showed known outcomes, demonstrating that PTML score correlates directly with progression-free survival (PFS) in patients who received pembrolizumab, with OS in patients with advanced melanoma receiving ipilimumab, and with both PFS and OS in patients receiving tumor-infiltrating lymphocyte therapy.41
Another independent prognostic factor for OS is baseline tumor burden (BTB), which may help guide physicians’ decisions in the selection of targeted therapies versus immunotherapies. BTB has been correlated with an increased probability of survival for patients wit advanced melanoma and large tumors that were treated with pembrolizumab, indicating that for patients with medium to large tumors, immunotherapies may be preferable over targeted therapies. Further analysis may aid in the optimization of dosing based on tumor size.42,43
NRAS (neuroblastoma RAS viral [v-ras] oncogene homolog, which encodes for a membrane protein involved in transport between the Golgi apparatus and plasma membrane) mutation status may also prove to be a valuable predictor of benefit with PD-1/PD-L1 pathway inhibition. NRAS mutation in melanoma represents a distinct cohort within the disease correlating to poor prognosis. Based on data from a retrospective chart review of 229 patients with melanoma receiving immunotherapies, it was discovered that NRAS-mutationpositive patients achieved greater benefit from PD-1/PD-L1 inhibition treatment compared with patients without the mutation. This suggests that NRAS mutation may be used as a selection factor for determination of patients that are optimal candidates for anti–PD-1 treatment.44
Analysis of biomarkers in patients treated with ipilimumab has also revealed that baseline lactate dehydrogenase (LDH; an enzyme that catalyzes the oxidation of lactate to pyruvate and vice versa) and the rate of increase in absolute lymphocyte count (ALC) are both positive biomarkers for greater benefit with CTLA-4 blockade.45,46It was subsequently revealed that there may be an ALC threshold of 1000 cells/μL, below which ipilimumab treatment may be ineffective.47
Biomarker Testing Paves the Way for Better Targeted Therapies in NSCLC
April 16th 2024At a live virtual event, Edward S. Kim, MD, MBA, discussed the evolving landscape of biomarker testing before making treatment decisions for patients with early-stage non–small cell lung cancer (NSCLC).
Read More
Creating Solutions for a 'Continual State of Transition' in Cancer Care
April 15th 2024In a Peers & Perspectives in Oncology feature article, we focus on the importance of the transition-of-care process for patients with cancer as they move from the inpatient to outpatient setting, as well as between lines of therapy with comments from Marc J. Braunstein, MD, PhD, and Michael Shusterman, MD.
Read More