Challenges in the Optimal Therapeutic Management of Geriatric Patients
Several recent studies have investigated the safety and efficacy of next-generation antiandrogen therapies and chemotherapy in the geriatric population.
1It is most frequently diagnosed in men age 65 to 74 years, with nearly 90% of all new cases diagnosed in men age ≥55 years.2
Given the demographic distribution of patients with prostate cancer, clinicians must consider and evaluate the unique characteristics and treatment options for this population. Unlike younger men, older men are more likely to be diagnosed with advanced disease, and tend to have higher mortality rates and poorer prognosis.3
Many older patients with prostate cancer are treated with systemic therapies, stressing the importance of evaluating clinical outcomes and safety profiles specifically in the elderly. Treating elderly patients presents certain challenges, including higher rates of comorbidities, physical frailty, and lower tolerance for adverse events (AEs). Several recent studies have investigated the safety and efficacy of next-generation antiandrogen therapies and chemotherapy in the geriatric population.
Studies With Abiraterone Acetate
Peter Mulders, MD, PhD, Radboud University Medical Center, Nijmegen, The Netherlands, and colleagues reported their findings of abiraterone acetate in elderly patients who had received prior chemotherapy in European Urology.4
In a post hoc analysis of the COU-AA-301 trial,5,6 the pivotal study that led to the approval of abiraterone acetate in patients who had received prior docetaxel, the efficacy and safety of abiraterone acetate (AA) plus prednisone was compared with placebo plus prednisone in younger (<75 years) and elderly (≥75 years) patients.
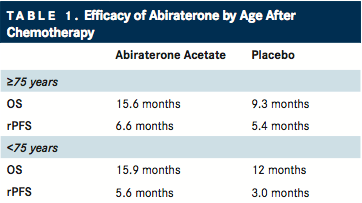
TABLE 1
Abiraterone acetate, the prodrug of abiraterone, is an inhibitor of CYP17A that targets extragonadal synthesis of androgen. Since 2011, it has been approved for use in patients with metastatic castration-resistant prostate cancer (mCRPC) who have received prior docetaxel therapy, and since 2012 for patients who are chemotherapy-naïve ().7
Compared with patients who received placebo plus prednisone, overall survival (OS) was significantly improved with AA plus prednisone in elderly patients (15.6 vs 9.3 months; P =.0022) and younger patients (15.9 vs 12.0 months; P =.0055). Similarly, AA plus prednisone significantly improved radiographic progression-free survival (PFS) in both elderly (6.6 vs 5.4 months; P =.0019) and younger (5.6 vs 3.0 months; P <.0001) patients, as well as time to prostate-specific antigen (PSA) progression (TTPP) in younger patients (8.4 vs 5.6 months; P <.0001).4
Grade 3/4 AEs were similar in both age groups. Rates of hypertension and hypokalemia, both associated with AA and mineralocorticoid excess, were greater in the AA-plusprednisone cohort, although rates were similar between age groups. Treatment-related cardiac events that led to study discontinuation were also similar between both treatment and age groups (1% vs 1%).5,6
“This analysis is reassuring with respect to the riskbenefit ratio of treating elderly patients with AA, and AA is a viable treatment option for the elderly, either after docetaxel or for those not fit for docetaxel due to other comorbidities,” the authors concluded.
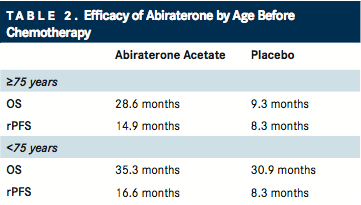
TABLE 2
Outcomes were further explored in an elderly population, in parallel post hoc analyses of the COU-AA-302 trial, which was the landmark study that led to the approval of AA in chemotherapy-naïve patients.8,9Further results were also published in the November 2015 issue of The Journal of Urology ().10
Clinical outcomes were significantly improved with AA plus prednisone compared with prednisone alone. For AA plus prednisone, radiographic PFS was superior in both elderly (14.9 vs 8.3 months; P =.0009) and younger (16.6 vs 8.3 months; P <.0001) cohorts, as well as OS in elderly patients (28.6 vs 25.6 months; P =.0268). All secondary endpoints also favored AA plus prednisone for both age groups. Adverse events associated with AA plus prednisone were similar between age groups.
Mineralocorticoid excess events (edema, hypokalemia, hypertension) occurred more frequently with AA plus prednisone than with prednisone alone in both elderly and younger patients. Although infrequent, grade 3 or 4 hepatoxicity and cardiac disorders were more frequent in both age cohorts with AA plus prednisone.
In conjunction with the results reported by Mulders et al, these findings “support the use of abiraterone therapy in elderly men with mCRPC regardless of prior chemotherapy,” Smith and coauthors wrote.
Comparable results have been observed in other studies. In one retrospective trial, Raya Leibowitz-Amit, MD, PhD, from Tel Aviv Hospital in Israel, and colleagues evaluated the toxicity and efficacy of AA and docetaxel in patients with mCRPC age ≥80 years, assessing PSA response rate, incidence of toxic AEs, OS, and biochemical PFS.11Relative to younger men, octogenarians had similar rates of PSA response rate, biochemical PFS, and OS with AA therapy. Treatment was well tolerated in both age groups, with no significant differences in mineralocorticoid excess events and mild liver toxicity rates.
The investigators stressed that despite this analysis being retrospective in nature, it reflects a “real life” experience because most data were obtained outside of a clinical trial. Taken together, the results of these studies support the use of AA in an elderly population, especially for patients who may be more sensitive to alternative therapies with greater toxicity.11
Studies With Enzalutamide
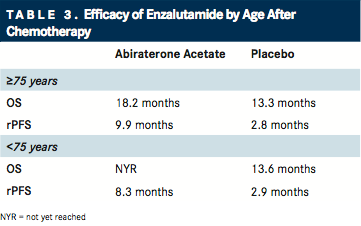
TABLE 3
In the February 2014 issue of Annals of Oncology, Cora Sternberg, MD, from San Camillo-Forlanini Hospital in Rome, Italy, and coauthors reported the outcomes of younger and elderly patients treated with enzalutamide who had received prior docetaxel therapy using a post hoc analysis of the AFFIRM trial (). Efficacy outcomes for OS, radiographic PFS, TTPP, and PSA response, as well as safety, were assessed.12,13
Enzalutamide is an androgen receptor inhibitor that competitively binds to its ligand-binding domain. It has been approved for use in patients with mCRPC both before and after docetaxel.
In both age cohorts, OS was significantly longer with enzalutamide compared with placebo (younger: median not reached vs 13.6 months; P <.0001; elderly: 18.2 vs 13.3 months; P = .004). Similarly, radiographic PFS was improved in younger (HR, 0.45; P <.001) and elderly (HR, 0.27; P <.001) patients with enzalutamide, as was TTPP and PSA response. The incidence of all-grade peripheral edema, fatigue, and diarrhea were greater in elderly patients than in younger patients with enzalutamide. Grade ≥3 AEs were similar between both age groups and treatment cohorts.13
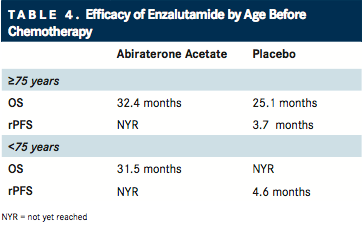
TABLE 4
In a more recent report in the February 2016 issue of Annals of Oncology,14Julie Graff, MD, from Oregon Health and Science University, and co-investigators analyzed the safety and efficacy of enzalutamide in younger and older patients with mCRPC who were chemotherapy-naïve using a post hoc analysis of the PREVAIL clinical trial ().15Among elderly patients, OS and radiographic PFS were significantly improved with enzalutamide compared with placebo. The incidence of AEs was similar between age groups and treatment groups, with the exception of falls, which was greater in elderly patients compared with younger patients, and in elderly patients treated with enzalutamide compared with placebo.
Investigators reasoned that the data from this study as well as previous post hoc analyses support that “secondgeneration hormone therapy is safe and effective in men aged ≥75 years, reinforcing the stance that physicians should not discriminate on the basis of age alone when selecting treatment for mCRPC.”
Enzalutamide does not require concomitant corticosteroid treatment. In terms of sequencing, the authors pointed out that AA and enzalutamide have unique safety profiles that should be carefully evaluated when making therapy selection for elderly patients.
Studies in Docetaxel
Until recent years, docetaxel was the only chemotherapeutic agent available that offered a survival advantage in mCRPC. Compared with mitoxantrone, docetaxel significantly improved OS and quality of life. However, toxicity in elderly patients, namely myelosuppression and sepsis, has been of concern. In the TAX327 trial, elderly patients age ≥75 years experienced significantly higher rates of infection and needed more docetaxel dose reductions than younger patients.16,17
To address these toxicity concerns, Hui-Li Wong, MD, from the Walter & Eliza Hall Institute of Medical Research in Sydney, Australia, and colleagues evaluated dosing practices, efficacy, and tolerability in very elderly patients (age ≥ 80 years) in a retrospective study of 20 patients with mCRPC.18 Although 56% of assessable patients had a PSA response rate of ≥50%, 40% required an initial dose reduction, and 55% required subsequent dose delays or reductions. Grade 3/4 hematologic toxicity occurred in 45% of patients, and chemotherapy-related complications requiring hospitalization occurred in 25% of patients.
Similarly, in the analysis by Leibowitz-Amit et al,11PSA response rates and OS were similar in octogenarians and younger men, although toxicity-related docetaxel cessation and febrile neutropenia occurred more frequently in octogenarians.
“Our results suggest that amelioration or prevention of chemotherapy-induced-toxicity is difficult in octogenarians even with modification of dose and schedule [with docetaxel], and that even awareness and careful vigilance of treating physicians cannot mitigate toxicity altogether,” Leibowitz-Amit and coauthors concluded.
Overall, advances in recent years have broadened the therapeutic options for all patients with mCRPC. In order to provide optimal clinical outcomes for elderly patients, clinicians must carefully weigh the individual needs of the patient against the safety and efficacy of specific agents, while remembering that elderly patients may have different goals of care than younger populations.
References
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66(1):7-30.
- National Cancer Institute. SEER Stat Fact Sheets: Prostate Cancer. 2016. http:// seer.cancer.gov/statfacts/html/prost.html. Accessed April 5, 2016.
- Scosyrev E, Messing EM, Mohile S, et al. Prostate cancer in the elderly: frequency of advanced disease at presentation and disease-specific mortality. Cancer. 2012;118(12):3062-3070.
- Mulders PFA, Molina A, Marberger M, et al. Efficacy and safety of abiraterone acetate in an elderly patient subgroup (aged 75 and older) with metastatic castration-resistant prostate cancer after docetaxel-based chemotherapy. Eur Urol. 2014;65(5):875-883.
- de Bono JS, Logothetis CJ, Molina A, et al. Abiraterone and increased survival in metastatic prostate cancer. N Engl J Med. 2011;364(21):1995-2005.
- Fizazi K, Scher HI, Molina A, et al. Abiraterone acetate for treatment of metastatic castration-resistant prostate cancer: final overall survival analysis of the COU-AA-301 randomised, double-blind, placebo-controlled phase 3 study. Lancet Oncol. 2012;13(10):983-992.
- Kessler ER, Flaig TW. Geriatric considerations in the treatment of advanced prostate cancer. F1000Prime Rep. 2014;6:33.
- Rathkopf DE, Smith MR, de Bono JS, et al. Updated interim efficacy analysis and long-term safety of abiraterone acetate in metastatic castration-resistant prostate cancer patients without prior chemotherapy (COU-AA-302). Eur Urol. 2014;66(5):815-825.
- Ryan CJ, Smith MR, Fizazi K, et al. Abiraterone acetate plus prednisone versus placebo plus prednisone in chemotherapy-naive men with metastatic castrationresistant prostate cancer (COU-AA-302): final overall survival analysis of a randomised, double-blind, placebo-controlled phase 3 study. Lancet Oncol. 2015;16(2):152-160.
- Smith MR, Rathkopf DE, Mulders PFA, et al. Efficacy and safety of abiraterone acetate in elderly (75 years or older) chemotherapy naïve patients with metastatic castration resistant prostate cancer. J Urol. 2015;194(5):1277-1284.
- Leibowitz-Amit R, Templeton AJ, Alibhai SM, et al. Efficacy and toxicity of abiraterone and docetaxel in octogenarians with metastatic castration-resistant prostate cancer. J Geriatr Oncol. 2015;6(1):23-28.
- Scher HI, Fizazi K, Saad F, et al. Increased survival with enzalutamide in prostate cancer after chemotherapy. N Engl J Med. 2012;367(13):1187-1197.
- Sternberg CN, de Bono JS, Chi KN, et al. Improved outcomes in elderly patients with metastatic castration-resistant prostate cancer treated with the androgen receptor inhibitor enzalutamide: results from the phase III AFFIRM trial. Ann Oncol. 2014;25(2):429-434.
- Graff JN, Baciarello G, Armstrong AJ, et al. Efficacy and safety of enzalutamide in patients 75 years or older with chemotherapy-naive metastatic castration-resistant prostate cancer: results from PREVAIL. Ann Oncol. 2016;27(2):286-294.
- Beer TM, Armstrong AJ, Rathkopf DE, et al. Enzalutamide in metastatic prostate cancer before chemotherapy. N Engl J Med. 2014;371(5):424-433.
- Petrylak DP, Tangen CM, Hussain MHA, et al. Docetaxel and estramustine compared with mitoxantrone and prednisone for advanced refractory prostate cancer. N Engl J Med. 2004;351(15):1513-1520.
- Tannock IF, de Wit R, Berry WR, et al. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med. 2004;351(15):1502-1512.
- Wong HL, Lok SW, Wong S, et al. Docetaxel in very elderly men with metastatic castration-resistant prostate cancer. Prostate Int. 2015;3(2):42-46.

Creating Solutions for a 'Continual State of Transition' in Cancer Care
April 15th 2024In a Peers & Perspectives in Oncology feature article, we focus on the importance of the transition-of-care process for patients with cancer as they move from the inpatient to outpatient setting, as well as between lines of therapy with comments from Marc J. Braunstein, MD, PhD, and Michael Shusterman, MD.
Read More