Steroid Use With Abiraterone Offers Multidimensional Benefits to Patients With mCRPC
For decades, the standard of care for men with advanced prostate cancer has been the depletion or inhibition of androgens. While androgen-deprivation therapy (ADT) often results in temporary tumor regression or symptom relief in some patients, disease progression ultimately occurs over time.
1For patients with metastatic disease, the median overall survival (OS), until very recently, had been less than 2 years after chemotherapy.2
While tumor progression with ADT was previously believed to be hormone-refractory or androgen-independent, a large body of evidence supports that metastatic castration-resistant prostate cancer (mCRPC) is commonly driven by elevated steroid synthesis, increased expression or splice variants of the androgen receptor (AR), or AR ligand promiscuity, indicating the ongoing need for targeted androgen therapies.3
Abiraterone acetate (AA), the prodrug of abiraterone, alone or in combination with low-dose steroids, has been intensely investigated in recent years for the treatment of progressive CRPC in patients who have received prior chemotherapy or who were treatment-naive. Abiraterone is a selective and potent inhibitor of cytochrome P450 c17 (CYP17), an enzyme that is critical for testosterone synthesis in the adrenal glands.4
Long-Term Analysis of Abiraterone Plus Prednisone
Despite the established clinical utility of steroids in mCRPC and other tumor types to manage adverse events (AEs) of chemotherapy and cancer-associated pain, long-term use of high doses of glucocorticoids, such as prednisone, have a number of associated AEs. As such, an analysis of the frequency and severity of AEs in patients treated with long-term dosing of AA and prednisone is of interest.
Data sets from the COU-AA-301 and COU-AA-302 phase III clinical trials were used to determine whether long-term use of low-dose prednisone with or without AA would lead to significant AEs in patients with mCRPC. Karim Fizazi, MD, PhD, from the Institut Gustave Roussy in Villejuif, France, and colleagues reported their findings in the March 7, 2016, issue of European Urology.5
Investigators analyzed AEs in 1195 patients from the COUAA-301 trial and 1088 patients from the COU-AA-302 trial. All patients received prednisone 5 mg twice daily for a median of 8.3 months. Investigators utilized the Standardized MedDRA Queries to identify preferred terms known for glucocorticoidassociated AEs.
For all patients, those treated with AA and prednisone (AA + P), and those treated with prednisone alone (P alone), the overall incidence of any-grade AEs for any prednisone exposure was 24.6%, 25.5%, and 23.3%, respectively. The most common any-grade AEs were hyperglycemia (7.4%, 7.8%, and 6.9% for all patients, those treated with AA + P, and those treated with P alone, respectively) and weight increase (4.3%, 3.9%, and 4.8%, respectively). Among any-grade AEs, ecchymosis, rib fracture, Cushingoid state, cataract, diabetes mellitus, and skin atrophy occurred at rates ≥1%. The incidence of grade ≥3 AEs for any prednisone exposure was 4.5%, 5.1%, and 3.7% for all patients, AA + P, and P alone, respectively. Hyperglycemia (48%) and cataract (10%) were the 2 most common grade ≥3 AEs. No discernable trends were observed in any-grade or grade ≥3 AEs when examined by duration of exposure.5
These results indicate that treatment with low-dose prednisone, administered with or without AA, is associated with few glucocorticoid-related AEs, and that the incidence of AEs is not directly related to prednisone exposure duration. As such, long-term treatment with AA plus prednisone is safe and tolerable for most patients.5
Charles Ryan, MD, from the University of California, San Francisco, and lead investigator of the COU-AA-302 study, stressed that the addition of prednisone to AA therapy has a triple purpose. “First, steroids have been traditionally used as a palliative treatment to control bone pain from the disease. Prednisone has always been included in the packets of treatments that included chemotherapy. That formed the basis for the FDA saying that prednisone is an acceptable treatment for some CRPC,” Ryan said. “Second, patients respond to steroids. The number of patients who have shown a clinical response to steroids alone is somewhere between 20% and 30%. So steroids have therapeutic efficacy as well as palliative benefits. Third, prednisone reduces the mineralocorticoid excess that can occur from abiraterone monotherapy. As such, AA is made safer with the addition of prednisone.”
Early phase I and II trials of AA demonstrated antitumor activity, and common AEs, such as hypokalemia, fluid retention, and hypertension associated with mineralocorticoid excess, were significantly reduced with co-treatement with low-dose corticosteroids, such as prednisone.68Given those initial positive results, additional phase III trials were completed using the AA-prednisone combination to determine its efficacy and safety in patients with CRPC.
In the phase III COU-AA-301 trial, patients with mCRPC who had received prior chemotherapy with docetaxel were treated with prednisone 5 mg twice daily plus AA 1000 mg or placebo to determine the effect on OS, time to prostatespecific antigen (PSA) progression (TTPP), and progressionfree survival (PFS). The initial results of the study were reported by Johann de Bono, MD, PhD, from the Institute of Cancer Research in London, and colleagues in The New England Journal of Medicine,9and the final analysis was reported by Fizazi and colleagues in The Lancet Oncology.10
Based on the final analysis of 1195 patients, all outcomes favored the AA treatment group. Compared with placebo, AA plus prednisone extended median OS (15.8 vs 11.2 months; P <.0001), median radiologic PFS (5.6 vs 3.6 months; P <.0001), median TTPP (8.5 vs 6.6 months; P <.0001), and proportion of patients with a PSA response (29.5% vs 5.5%; P <.0001).10 Mineralocorticoid-related AEs were more common in the AA-plus-prednisone group than with placebo plus prednisone, although grade 3/4 AEs were similar between treatment groups.9,10
The phase III COU-AA-302 examined the safety and efficacy of AA plus prednisone in 1088 patients with mCRPC without prior chemotherapy. Patients were treated in a similar fashion as in the COU-AA-301 study. The primary outcomes were radiologic PFS and OS. Interim results were reported by Dana Rathkopf, MD, from Memorial Sloan Kettering Cancer Center in New York City, and colleagues in European Urology,11 and the final analysis reported by Charles Ryan, MD, from the UCSF Helen Diller Family Comprehensive Cancer Center, and colleagues in The Lancet Oncology.12
Analysis of Varying Dosing of Glucocorticoids
After an extended follow-up period, patients who had received AA plus prednisone had significantly longer median OS (34.7 vs 30.3 months; P =.0033) and PFS (16.5 vs 8.2 months; P <.0001) than with placebo.11,12Similar to the COUAA-301 trial, AEs related to mineralocorticoid excess were more common with AA than with placebo, although most were grade 1 or 2 in severity.
Ongoing clinical trials are evaluating various dosing and glucocorticoid combinations with AA in patients with mCRPC to determine the incidence of AEs associated with mineralocorticoid excess. Gerhardt Attard, MD, PhD, from the Institute of Cancer Research in London, and colleagues presented their findings at the 2016 Genitourinary Cancers Symposium.13
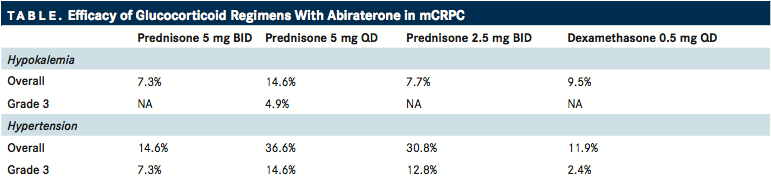
TABLE
In the phase II trial, 164 patients with asymptomatic chemotherapy-naive mCRPC were randomized to receive AA 1000 mg once daily plus prednisone at 1 of 3 different dosing schedules or dexamethasone at 1 dosing schedule. The primary endpoint was the percentage of patients who experienced hypokalemia or hypertension during the first 24 weeks of treatment. In comparison with patients who received prednisone 5 mg once daily or 2.5 mg twice daily, patients who received prednisone 5 mg twice daily and dexamethasone 0.5 mg once daily had lower rates of overall hypokalemia (14.6% and 7.7% vs 7.3% and 9.5%, respectively) and hypertension (36.6% and 30.8% vs 14.6% and 11.9%, respectively). A similar trend was observed for grade 3 AEs, and there were no grade 4 events ().13
These data indicate that the approved dosing of prednisone 5 mg twice daily with AA or dexamethasone 0.5 mg once daily can adequately prevent mineralocorticoid excess‒associated AEs. However, additional research will be needed before there is acceptance of a lower prednisone strategy.
In summary, the long-term use of prednisone with AA appears to be safe and well tolerated in patients with mCRPC. Additionally, the use of prednisone offers multidimensional benefits. “Many patients with advanced prostate cancer have a pain component to their disease,” Ryan stated. “We need to remember that prednisone can help to manage some of the pain issues. It does continue to provide a palliative benefit, and it probably contributes to the clinical benefit of abiraterone overall.”
References
- Crawford ED. Understanding the epidemiology, natural history, and key pathways involved in prostate cancer. Urology. 2009;73(5 suppl):S4-10.
- Halabi S, Small EJ, Kantoff PW, et al. Prognostic model for predicting survival in men with hormone-refractory metastatic prostate cancer. J Clin Oncol. 2003;21(7):1232- 1237.
- Massard C, Fizazi K. Targeting continued androgen receptor signaling in prostate cancer. Clin Cancer Res. 2011;17(12):3876-3883.
- Attard G, Belldegrun AS, De Bono JS. Selective blockade of androgenic steroid synthesis by novel lyase inhibitors as a therapeutic strategy for treating metastatic prostate cancer. BJU Int. 2005;96(9):1241-1246.
- Fizazi K, Chi KN, de Bono JS, et al. Low incidence of corticosteroid-associated ad- verse events on long-term exposure to low-dose prednisone given with abiraterone acetate to patients with metastatic castration-resistant prostate cancer [published online March 7, 2016]. Eur Urol. doi: 10.1016/j.eururo.2016.02.035.
- Danila DC, Morris MJ, Bono JS de, et al. Phase II multicenter study of abiraterone ac- etate plus prednisone therapy in patients with docetaxel-treated castration-resistant prostate cancer. J Clin Oncol. 2010;28(9):1496-1501.
- Reid AHM, Attard G, Danila DC, et al. Significant and sustained antitumor activity in post-docetaxel, castration-resistant prostate cancer with the CYP17 inhibitor abiraterone acetate. J Clin Oncol. 2010;28(9):1489-1495.
- Attard G, Reid AHM, A’Hern R, et al. Selective inhibition of CYP17 with abiraterone acetate is highly active in the treatment of castration-resistant prostate cancer. J Clin Oncol. 2009;27(23):3742-3748.
- de Bono JS, Logothetis CJ, Molina A, et al. Abiraterone and increased survival in metastatic prostate cancer. N Engl J Med. 2011;364(21):1995-2005.
- Fizazi K, Scher HI, Molina A, et al. Abiraterone acetate for treatment of metastatic castration-resistant prostate cancer: final overall survival analysis of the COU- AA-301 randomised, double-blind, placebo-controlled phase 3 study. Lancet Oncol. 2012;13(10):983-992.
- Rathkopf DE, Smith MR, de Bono JS, et al. Updated interim efficacy analysis and long-term safety of abiraterone acetate in metastatic castration-resistant prostate cancer patients without prior chemotherapy (COU-AA-302). Eur Urol. 2014;66(5):815-825.
- Ryan CJ, Smith MR, Fizazi K, et al. Abiraterone acetate plus prednisone versus pla- cebo plus prednisone in chemotherapy-naive men with metastatic castration-resis- tant prostate cancer (COU-AA-302): final overall survival analysis of a randomised, double-blind, placebo-controlled phase 3 study. Lancet Oncol. 2015;16(2):152-160.
- Attard G, Merseburger AS, Sternberg CN, et al. A randomized trial of abiraterone acetate (AA) administered with 1 of 4 glucocorticoid (GC) regimens in metastatic castration-resistant prostate cancer (mCRPC) patients (pts). J Clin Oncol. 2016;34(2_ suppl; abstr 261).

Novel Approaches Focus on Limiting Toxicity in Older Patients With ALL
April 22nd 2024The major challenges for clinicians treating older patients with acute lymphoblastic leukemia surround the emergence of resistance to existing therapies and the toxicities associated with current chemotherapies.
Read More
Ornstein Advises on Starting Dose and Management of Lenvatinib in RCC
April 21st 2024During a Case-Based Roundtable® event, Moshe Ornstein, MD, MA, provided guidance on dosing and toxicity concerns in a patient treated with lenvatinib plus pembrolizumab for advanced renal cell carcinoma.
Read More
Rugo Surveys Peers on Treatment After Metastatic Relapse of HR+, HER2– Breast Cancer
April 20th 2024During a Case-Based Roundtable® event, Hope S. Rugo, MD, FASCO, moderated a discussion on treatment options for a patient whose breast cancer recurred several years after adjuvant therapy.
Read More