Emerging Treatment Strategies for Nonsquamous Non-Small Cell Lung Cancer
Although great advances have been made in the treatment of advanced, metastatic, and nonresectable, nonsquamous, non–small cell lung cancer (NSCLC), prognosis remains relatively poor, and recurrence is common. Howard Jack West, MD, medical director of the Thoracic Oncology Program at Swedish Cancer Institute, Seattle, Washington, explained in an abstract that “platinum-based chemotherapy is the current standard of care for patients with newly diagnosed advanced nonsquamous NSCLC.
1
The Evolving Components of Combination Therapies
For some patient populations, such as those with high PD-L1 expression, single-agent immunotherapy may be an option; however, for the majority of patients with nonsquamous NSCLC, combination therapy is essential for successful first-line treatment. PD-1 and PD-L1 frequently serve as the backbone of combination immunotherapy regimens. According to Patrick Ott, MD, PhD, of the Dana-Farber Cancer Institute, Boston, Massachusetts, checkpoint inhibitors are favorable because “the established antitumor activity of PD-1/PD-L1 inhibition as monotherapy in a wide spectrum of cancers coupled with its favorable toxicity profile provides a strong rationale for its use as a backbone for combinatorial strategies. Despite the vastly accelerated pace of preclinical and clinical investigation of other cancer immunotherapy agents in recent years, this combination of broad single-agent activity and tolerability seen with PD-1 pathway inhibition is so far unparalleled; there are no other compounds on the horizon that could take the place of PD-1 pathway inhibition for this purpose.”2
Several ongoing and upcoming trials are investigating the combination of immunotherapy with chemotherapy regimens. Chemotherapy is already a part of the approved first-line pembrolizumab combination treatment regimen. Explaining the rationale behind chemotherapy and immunotherapy combinations, Ott wrote, “Chemotherapy-induced cancer cell death can promote tumor antigen presentation, potentially leading to priming of tumor-specific T cells in addition to its capacity to directly stimulate immune effectors and inhibit immune suppressive factors. Therefore, chemotherapy has the potential to convert a non-inflamed tumor into an inflamed one and may thus lead to synergy with PD-1/PD-L1 inhibition particularly in non-inflamed, chemotherapy-sensitive tumors.”2
Pemetrexed, the folic acid analog, is often used in conjunction with carboplatin or cisplatin to form the chemotherapy component of combination immunotherapy. This is primarily due to the favorable safety profile of pemetrexed compared with other options. Martin Edelman, MD, a medical oncologist and chair of the Department of Hematology/Oncology at Fox Chase Cancer Center, Philadelphia, Pennsylvania, noted that a clinical trial evaluating pemetrexed and gemcitabine found "highly significant advantages for the pemetrexed arm in both hematologic and nonhematologic toxicities."3,4
Another emerging strategy in combination immunotherapy is the use of PD-L1 inhibitors with "the blockade of the non-redundant and complementary checkpoint (cytotoxic T lymphocyte-associated protein 4 [CTLA-4])," according to Ott.2By targeting multiple immune checkpoints from different angles, the immunotherapy outcomes may be amplified.2Ipilimumab (Yervoy) is the main CTLA-4 inhibitor being investigated for combination treatment of nonsquamous NSCLC, although tremelimumab is also under investigation in combination with durvalumab (Imfinzi).5Combining PD-L1 inhibitors with CTLA-4 inhibitors may lead to an increase in immune-related adverse events (AEs).2
Confirming Efficacy and Safety of Pembrolizumab Plus Pemetrexed/Carboplatin
Pembrolizumab (Keytruda) in combination with pemetrexed and carboplatin received accelerated approval from the FDA for patients with nonsquamous NSCLC regardless of PD-L1 expression levels.6The phase II trial upon which the approval was based evaluated partial responses and progression-free survival (PFS), but the FDA required further evaluation using clinically meaningful endpoints, such as overall survival (OS). Corey Langer, MD, director of Thoracic Oncology at the Hospital of the University of Pennsylvania in Philadelphia and senior author on the phase III KEYNOTE-021 study, explained that the accelerated approval of first-line combination pembrolizumab “is an important milestone, but raises a number of issues since it occurred in the absence of phase III data or a proven survival advantage and was based on a relatively small phase II trial with just 120 enrollees. In this regard, we await the results of the ongoing phase III trial, KEYNOTE-189.”7
KEYNOTE-1898 is ongoing and will compare pembrolizumab plus chemotherapy with chemotherapy alone in 570 chemotherapy-naïve patients with an ECOG performance status (PS) of 0 or 1. After 4 initial cycles of combination therapy or chemotherapy alone, patients will continue pembrolizumab for 35 cycles or until disease progression, intolerable toxicity, or withdrawal. For patients who progress on chemotherapy alone, crossover to pembrolizumab is allowed. Patients will be stratified by smoking status, cisplatin or carboplatin, and PD-L1 status (FIGURE 1).8Per Langer, “the continued approval of pembrolizumab frontline with chemotherapy will depend on successful outcomes in KEYNOTE-189.”7

In research presented at the 2017 World Conference on Lung Cancer, the safety results from cohort G of KEYNOTE-021 as well as from PRONOUNCE and PARAMOUNT trials were evaluated in a descriptive analysis.9The 3 included trials studied first-line treatment of NSCLC using pemetrexed-based chemotherapy with or without pembrolizumab. The proportion of patients who completed all 4 cycles of induction therapy ranged from 70.8% in PRONOUNCE to 88.1% in the combination-therapy arm in cohort G of KEYNOTE-021. The median number of treatment cycles were 6 and 11 in PRONOUNCE and cohort G of KEYNOTE-021, respectively. Although AEs occurred more frequently in the pembrolizumab groups, the researchers wrote that the safety profile was “reasonable and manageable” for the pemetrexed combinations with or without immunotherapy. They concluded that the “ongoing randomized studies of the combination could further inform the safety/efficacy of pemetrexed/platinum plus immunotherapy.”9
First-Line Nivolumab Combination Regimens: CheckMate Studies
Nivolumab (Opdivo) is currently being investigated as a first-line combination treatment for advanced NSCLC after showing no benefit as a single-agent therapy for patients with elevated PD-L1 expression.10In CheckMate-026,11 nivolumab did not confer a benefit for PFS (HR, 1.15; P = .25) or for OS (HR, 1.02) compared with platinum-based chemotherapy in patients with 5% or higher PD-L1 expression. David Carbone, MD, PhD, professor of medicine at The Ohio State University Comprehensive Cancer Center, in Columbus, Ohio, explained in a news release that "the good news is that we discovered that a subset of patients who had both high tumor mutation burden and high PD-L1positive status did experience a significant benefit from immunotherapy."12
In the phase I CheckMate 012 study,13nivolumab with or without ipilimumab was tested as first-line treatment in advanced NSCLC. Patients with untreated stage IIIb/IV NSCLC were randomly assigned to receive nivolumab plus ipilimumab at varying time points. Compared with nivolumab plus ipilimumab every 6 weeks, nivolumab plus ipilimumab every 12 weeks resulted in a better objective response rate (ORR; 47% vs 38%). Moreover, the study authors reported that the response appeared to be durable, as a median duration of response (DoR) was not achieved in either cohort, with a median follow-up of 12.8 months in the 12-week cohort and 11.8 months in the 6-week cohort. Grade 3/4 AEs were reported in 37% of the 12-week cohort and 33% of the 6-week cohort.13After longer follow-up, updated results revealed that PFS in the pooled combination cohorts who had PD-L1 expression of at least 1% had a median PFS of 12.7 months; median PFS was still not reached for those patients with at least 50% expression. Furthermore, 1-year OS was 100% for patients with at least 50% PD-L1 expression in the pooled combination cohorts.14In a press release, Scott N. Gettinger, MD, associate professor of medicine at Yale Cancer Center, New Haven, Conneticut, stated, “We look forward to further evaluating [nivolumab] plus [ipilimumab] in the first- line treatment setting for advanced lung cancer.” Based on these results, and given the favorable toxicity profile of nivolumab, the agent is now being tested as a first-line combination therapy in CheckMate 227.10
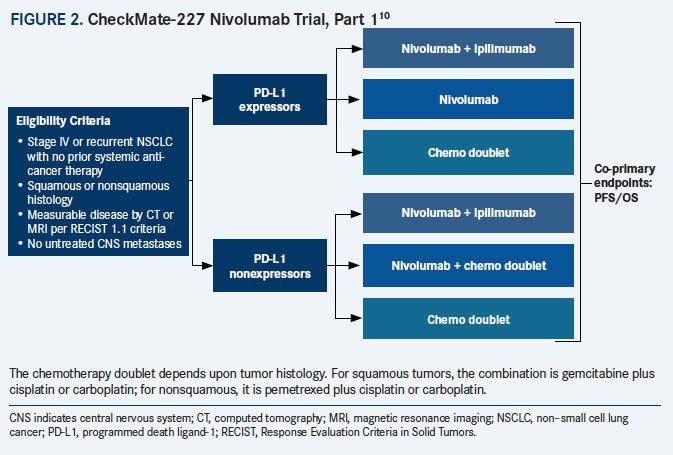
Part 1 of CheckMate 227, which is ongoing and has completed patient enrollment, includes adult patients with stage IV/recurrent NSCLC with no previous exposure to systemic therapy. Patients with at least 1% PD-L1 expression were randomly assigned to receive nivolumab every 2 weeks, nivolumab every 2 weeks plus ipilimumab every 6 weeks, or chemotherapy. Patients who were negative for PD-L1 expression were randomized to receive nivolumab plus ipilimumab, nivolumab plus chemotherapy, or chemotherapy alone. In part 2 of CheckMate 227, patients with advanced NSCLC across the PD-L1 expression spectrum will be randomized to receive platinum-based chemotherapy with or without nivolumab (FIGURE 2).10
A 3-year analysis of nivolumab in combination with chemotherapy from the CheckMate 012 trial was recently reported at the World Conference on Lung Cancer 2017.15In this study, participants were randomly assigned to receive nivolumab in combination with gemcitabine plus cisplatin (squamous only); pemetrexed plus cisplatin (nonsquamous only); or paclitaxel plus carboplatin (any histology). The median duration of chemotherapy was approximately 12 weeks, whereas the median duration of nivolumab treatment across cohorts was between 17 and 22 weeks. After induction therapy, patients were allowed to continue receiving nivolumab until progression. Across all cohorts, the median duration of chemotherapy treatment was 12 weeks, and the median duration of nivolumab treatment was between 7 and 22 weeks. Nivolumab with chemotherapy resulted in an ORR of 46%, median PFS of 6.0 months, median OS of 19.2 months, and a 3-year OS rate of 25%. No differences were noted between PD-L1negative and –positive groups for ORRs (48% vs 52%) or for OS (19.2 vs 20.2 months). According to the researchers, “these results support further evaluation of nivolumab-chemotherapy combinations as first-line treatment for advanced NSCLC.”15
Atezolizumab Combination Therapies: IMpower Studies
Atezolizumab (Tecentriq), a PD-L1 inhibitor, has been shown to be effective as a monotherapy compared with historic controls in patients with at least 5% PD-L1 expression in the phase II BIRCH trial.16For patients who were receiving atezolizumab as a first-line therapy, the median OS was 23.5 months. As a second- and third-line therapy, atezolizumab conferred median OS of 15.5 and 13.2 months, respectively. The researchers also noted that the OS was higher among patients with the highest levels of PD-L1 expression.16In the POPLAR trial,17atezolizumab monotherapy was compared with docetaxel chemotherapy. Overall survival was similar between the 2 treatments for patients who were PD-L1negative (HR, 1.04; P = .871), but was substantially improved in the atezolizumab group among patients who had 50% or higher PD-L1 expression (HR, 0.49; P = .068) and 5% or higher PD-L1 expression (HR, 0.54; P = .014).17To investigate atezolizumab in the first-line setting, multiple combinatorial approaches are being tested in ongoing phase III clinical trials.
In the IMpower 132 study,1approximately 568 patients with stage IV NSCLC and no previous chemotherapy will be randomly assigned to receive platinum-based chemotherapy plus pemetrexed with or without atezolizumab. Patients are being enrolled regardless of PD-L1 status, but the analysis of the data will include stratification based on PD-L1 expression data as well as sex, carboplatin compared with cisplatin, and smoking status. Among patients who do not progress, treatment with atezolizumab (in the atezolizumab group) or pemetrexed (in the chemotherapy-only group) will be continued until progression. The primary outcome measures will be PFS and OS.1
The efficacy of bevacizumab in combination with atezolizumab is being investigated in the IMpower 150 trial.18For this study, patients with NSCLC will be randomly assigned to receive atezolizumab with paclitaxel and carboplatin, atezolizumab plus bevacizumab with paclitaxel and carboplatin, or bevacizumab with paclitaxel and carboplatin. Participants will be PS 0 or 1 with stage IV NSCLC and no previous treatment with a checkpoint inhibitor. The primary outcome measures will be PFS and OS.
Ongoing Durvalumab Clinical Trials
Durvalumab is a PD-L1 inhibitor that is currently being investigated as a monotherapy and as a combination therapy for nonsquamous NSCLC, based on the results of the phase II ATLANTIC clinical trial.19In this study, patients with stage IIIB/IV NSCLC who had at least 2 prior systemic treatment regimens and PS of 0 or 1 received durvalumab. The ORR was higher among patients with PD-L1 expression of 25% or higher compared with those with lower PD-L1 expression (16.4% vs 7.5%). Among patients with PD-L1 expression levels of 90% or greater, the ORR was 30.9%. Most AEs were not severe, with 10.2% of patients experiencing grade 3/4 AEs.19The studyʼs first author, Marina C. Garassino, MD, medical director at the IRCCS Istituto Nazionale dei Tumori Foundation, Milan, Italy, concluded that, "Results are consistent with other anti[PD-1] and anti–PD-L1 compounds in metastatic NSCLC. We are awaiting the final results of several phase III trials…to clarify the role of durvalumab alone or in combination in NSCLC."20The ongoing phase III trials include MYSTIC and NEPTUNE, which compare durvalumab with or without tremelimumab and chemotherapy.
In the phase III MYSTIC trial,21systemic treatmentnaïve patients with stage IV NSCLC were randomly assigned to receive durvalumab, durvalumab plus tremelimumab, or standard-of-care chemotherapy. Durvalumab plus tremelimumab failed to achieve 1 of its co-primary endpoints: improved PFS relative to chemotherapy in patients with PD-L1 expression of at least 25%. According to a press release detailing the PFS results, durvalumab monotherapy would have improved PFS relative to chemotherapy, but this was not a prespecified endpoint.21In the same press release, Sean Bohen, executive vice president of Global Medicines Development and chief medical officer at AstraZeneca, explained, “While the results from the MYSTIC trial for progression-free survival in first-line stage IV [NSCLC] compared with standard of care are disappointing, the trial was designed to assess overall survival, and we look forward to evaluating the remaining primary endpoints of overall survival for both mono- and combination therapy.”21The OS results are expected in early 2018.21
Like MYSTIC, NEPTUNE is a phase III study evaluating durvalumab plus tremelimumab compared with standard of care, but there is no monotherapy arm. In this ongoing study, approximately 800 systemic treatmentnaïve patients with stage IV NSCLC will be randomly assigned to receive durvalumab plus tremelimumab or platinum-based chemotherapy. Patients will be stratified by PD-L1 status, histology, and smoking. OS is the primary endpoint, and secondary endpoints include PFS, ORR, DoR, and 1-year survival.5
Durvalumab in combination with platinum-based chemotherapy and with or without tremelimumab has been tested in a phase Ib study, with pemetrexed and durvalumab continued after induction therapy.22A total of 24 patients were enrolled, and most AEs were grade 2 or lower. Among the 17 patients who were evaluable for response, the ORR was 52.9%. According to the authors, more studies utilizing durvalumab and platinum-based chemotherapy are planned.22
Immunomodulatory Effects of Pemetrexed With Immunotherapy
Mechanistic studies are now attempting to determine whether pemetrexed synergizes with checkpoint inhibitors, and if so, through what pathways. In research presented at the 2017 World Conference on Lung Cancer, Ruslan Novosiadly, MD, PhD,senior research adviser for cancer immunobiology at Eli Lilly and Company, used a mouse model to “understand the effects of pemetrexed on tumor immune microenvironment and evaluate its efficacy in combination with PD-1 pathway inhibition.”23
In this study, mice with syngeneic MC38 tumors were treated with pemetrexed with or without cisplatin or carboplatin. Some tumors were also treated with antimouse PD-L1 antibody. The researchers evaluated the immune cell subsets, immune-related gene expression changes in the tumor tissue, and energy metabolism.23The researchers reported that pemetrexed treatment increased the number of intratumoral leukocytes, increased the number of intratumoral T-cells, and upregulated immune-related genes related to antigen presentation and T-cell infiltration and activation. In contrast, the immune gene expression signatures were not affected by the presence of cisplatin or carboplatin. The energy metabolism of T cells may have also been affected by pemetrexed exposure. The study authors noted that the combination of pemetrexed with antiPD-L1 slowed tumor growth compared with single-agent treatments. The authors concluded, “Pemetrexed exerts positive effects on the intratumor T-cell–mediated immune response independently of platinum agents, and enhances effects of PD-L1 antibody in MC38 syngeneic mouse tumor model.”23
Based on the multitude of ongoing and upcoming phase III trials, combination immunotherapy is a promising emerging treatment strategy.2More research will be needed to determine the mechanisms by which different agents work in concert to exert synergistic effects in patients with NSCLC.
References:
- West HJ, Nishio M, Dols MC, et al. IMpower132: a phase III clinical program1L atezolizumab plus platinum-based chemotherapy in chemo-naive advanced non-squamous NSCLC.J Clin Oncol.2017;35(suppl 15; abstr TPS9101).
- Ott PA, Hodi FS, Kaufman HL, et al. Combination immunotherapy: a road map.J Immunother Cancer.2017;5:16. doi: 10.1186/s40425-017-0218-5.
- Mulcahy N. Not legit? The superiority of pemetrexed in lung cancer. Medscape website. medscape.com/viewarticle/883481. Medscape. Published July 27, 2017. Accessed October 23, 2017.
- Scagliotti GV, Parikh P, von Pawel J, et al. Phase III study comparing cisplatin plus gemcitabine with cisplatin plus pemetrexed in chemotherapy-naive patients with advanced-stage non-small-cell lung cancer.J Clin Oncol.2008;26(21):3543-3551. doi: 10.1200/JCO.2007.15.0375.
- Mok T, Schmid P, Aren O, et al. 192TiP: NEPTUNE: a global, phase 3 study of durvalumab (MEDI4736) plus tremelimumab combination therapy versus standard of care (SoC) platinum-based chemotherapy in the first-line treatment of patients (pts) with advanced or metastatic NSCLC.J Thorac Oncol.2016;11(suppl 4):S140-S141. doi: 10.1016/S1556-0864(16)30301-X.
- FDA approves Merck’s KEYTRUDA® (pembrolizumab) as first-line combination therapy with pemetrexed and carboplatin for patients with metastatic nonsquamous non-small cell lung cancer (NSCLC), irrespective of PD-L1 expression [news release]. Rahway, NJ: Merck & Co, Inc; May 10, 2017. merck.com/news/press-release-details/2017/FDA-Approves-Mercks-KEYTRUDA-pembrolizumab-as-First-Line-Combination-Therapy-with-Pemetrexed-and-Carboplatin-for-Patients-with-Metastatic-Nonsquamous-Non-Small-Cell-Lung-Cancer-NSCLC-Irrespective-of-PD-L1-Expression/default.aspx. Accessed October 23, 2017.
- Inman S. Pembrolizumab/chemo PFS benefit in NSCLC sustained with longer follow-up. OncLive website. Published June 3, 2017. www.onclive.com/conference-coverage/asco-2017/pembrolizumab-chemo-pfs-benefit-in-nsclc-sustained-with-longer-followup. June 3, 2017. Accessed October 23, 2017.
- Hall RD, Gadgeel SM, Garon EB, et al. Phase 3 study of platinum-based chemotherapy with or without pembrolizumab for first-line metastatic, nonsquamous non-small cell lung carcinoma (NSCLC): KEYNOTE-189.J Clin Oncol.2016;3(suppl 15; abstr TPS9104).
- Govindan R, Borghaei H, He S, et al. Pemetrexed plus platinum chemotherapy with or without immunotherapy in non-squamous NSCLC: descriptive safety analysis. Presented at: 18th World Conference on Lung Cancer; October 15-18, 2017; Yokohama, Japan. Abstract P2.01-040.
- Paz-Ares L, Brahmer J, Hellmann MD, et al. CheckMate 227: a randomized, open-label phase 3 trial of nivolumab, nivolumab plus ipilimumab, or nivolumab plus chemotherapy versus chemotherapy in chemotherapy-naïve patients with advanced non-small cell lung cancer (NSCLC).Ann Oncol.2017;28(suppl_2; abstr 144TiP. doi.org/10.1093/annonc/mdx091.064.
- Carbone DP, Reck M, Paz-Ares L, et al; CheckMate 026 investigators. First-line nivolumab in stage IV or recurrent non-small-cell lung cancer.N Engl J Med.2017;376(25):2415-2426. doi: 10.1056/NEJMoa1613493.
- First-line immunotherapy treatment can improve survival for subset of lung cancer patients [news release]. Columbus, OH: Ohio State University; June 21, 2017. https://cancer.osu.edu/news-and-media/news/first-line-immunotherapy-treatment-can-improve-survival-for-subset-of-lung-cancer-patients. Accessed October 23, 2017.
- Hellmann MD, Rizvi NA, Goldman JW, et al. Nivolumab plus ipilimumab as first-line treatment for advanced non-small-cell lung cancer (CheckMate 012): results of an open-label, phase 1, multicohort study.Lancet Oncol.2017;18(1):31-41. doi: 10.1016/S1470-2045(16)30624-6.
- Encouraging survival observed with Opdivo (nivolumab) plus Yervoy (ipilimumab) with longer follow-up in first-line advanced non-small cell lung cancer, in updated phase 1b CheckMate -012 study [press release]. New York City: Bristol-Myers Squibb; 2016. news.bms.com/press-release/bmy/encouraging-survival-observed-opdivo-nivolumab-plus-yervoy-ipilimumab-longer-follo. December 5, 2016. Accessed October 23, 2017.
- Juergens R, Hellman M, Brahmer J, et al. First-line nivolumab plus platinum-based doublet chemotherapy for advanced NSCLC: CheckMate 012 3-year update. Presented at :18th World Conference on Lung Cancer; October 15-18, 2017; Yokohama, Japan. Abstract OA 17.03.
- Peters S, Gettinger S, Johnson ML, et al. Phase II trial of atezolizumab as first-line or subsequent therapy for patients with programmed death-ligand 1selected advanced non–small-cell lung cancer (BIRCH).J Clin Oncol.2017;35(24):2781-2789. doi: 10.1200/JCO.2016.71.9476.
- Fehrenbacher L, Spira A, Ballinger M, et al. Atezolizumab versus docetaxel for patients with previously treated non-small-cell lung cancer (POPLAR): a multicentre, open-label, phase 2 randomised controlled trial.Lancet.2016;387(10030):1837-1846. doi: 10.1016/S0140-6736(16)00587-0.
- A study of atezolizumab in combination with carboplatin plus (+) paclitaxel with or without bevacizumab compared with carboplatin+paclitaxel+bevacizumab in participants with stage IV non-squamous non-small cell lung cancer (NSCLC) (IMpower150). clinicaltrials.gov/ct2/show/NCT02366143. Updated October 11, 2017. Accessed October 23, 2017.
- Garassino M, Vansteenkiste J, Kim J-H, et al. PL04a.03: durvalumab in 3rd-line locally advanced or metastatic, EGFR/ALK wild-type NSCLC: results from the phase 2 ATLANTIC study.J Thorac Oncol.12(1):S10-S11. doi: 10.1016/j.jtho.2016.11.012.
- Durvalumab shows activity in heavily pretreated patients with nonsmall cell lung cancer. ASCO Post website. ascopost.com/issues/february-25-2017-ce-supplement-iaslc-immunotherapies-for-nsclc/durvalumab-shows-activity-in-heavily-pretreated-patients-with-non-small-cell-lung-cancer/. Published February 25, 2017. Accessed October 23, 2017.
- AstraZeneca reports initial results from the ongoing MYSTIC trial in stage IV lung cancer [news release]. Cambridge, UK: AstraZeneca. July 27, 2017. astrazeneca.com/media-centre/press-releases/2017/astrazeneca-reports-initial-results-from-the-ongoing-mystic-trial-in-stage-iv-lung-cancer-27072017.html. Accessed October 23, 2017.
- Juergens R, Hao D, Laurie S, et al. MA09.03 Cisplatin/pemetrexed + durvalumab +/- tremelimumab in pts with advanced non-squamous NSCLC: a CCTG phase IB study - IND.226.J Thorac Oncol.2017;12(1):S392-S393. doi: 10.1016/ j.jtho.2016.11.445.
- Novosiadly R, Schaer D, Lu Z, et al. Pemetrexed exerts intratumor immunomodulatory effects and enhances efficacy of immune checkpoint blockade in MC38 syngeneic mouse tumor model. Presented at: World Conference of Lung Cancer; October 15-18, 2017; Yokohama, Japan. Abstract P3.07-006.

Novel Approaches Focus on Limiting Toxicity in Older Patients With ALL
April 22nd 2024The major challenges for clinicians treating older patients with acute lymphoblastic leukemia surround the emergence of resistance to existing therapies and the toxicities associated with current chemotherapies.
Read More
Ornstein Advises on Starting Dose and Management of Lenvatinib in RCC
April 21st 2024During a Case-Based Roundtable® event, Moshe Ornstein, MD, MA, provided guidance on dosing and toxicity concerns in a patient treated with lenvatinib plus pembrolizumab for advanced renal cell carcinoma.
Read More
Peers Discuss Role of Pola-R-CHP vs R-CHOP in Newly Diagnosed DLBCL
April 19th 2024During a Case-Based Roundtable® event, Haifaa Abdulhaq, MD discussed with participants whether the POLARIX trial influences their choice to use the pola-R-CHP as opposed to R-CHOP regimen for patients with newly diagnosed diffuse large B-cell lymphoma.
Read More