A Primer on the Epidemiology and Pathophysiology of Cutaneous Squamous Cell Carcinoma
Cutaneous squamous cell carcinoma is the second most common form of skin cancer, with an estimated 1 million cases treated in the United States each year. Although most cSCCs are localized and can be easily treated, approximately 5% of patients will experience local recurrence, 4% will develop nodal metastases, and up to 2% will die of the disease. In addition to the small but significant number of deaths attributed to cSCC each year, the disease and its associated precancerous skin lesions contribute to a large financial burden of more than $4.5 billion annually in the United States.
1Although most cSCCs are localized and can be easily treated, approximately 5% of patients will experience local recurrence, 4% will develop nodal metastases, and up to 2% will die of the disease.2In addition to the small but significant number of deaths attributed to cSCC each year, the disease and its associated precancerous skin lesions contribute to a large financial burden of more than $4.5 billion annually in the United States.3
cSCC is a type of nonmelanoma skin cancer (NMSC), the most frequently diagnosed cancer in North America.4 In 2006, the estimated number of incident cases of NMSC in the United States was 4,013,890, and approximately 62% of affected individuals were treated for ≥1 skin cancer.1In 2012, the estimated number of NMSCs in the United States was 5,434,193, and 3,315,554 individuals were treated.1Of these patients with NMSCs, there were estimated to be 1,027,700 cases of cSCC.1Individuals with lighter skin have been diagnosed with cSCCs at a significantly higher rate than those with darker skin types.5
Epidemiology
Several causes of cSCC have been described, such as ultraviolet (UV) radiation; ionizing radiation; genodermatoses; human papillomavirus (HPV); arsenic; polycyclic aromatic hydrocarbons; immunosuppression; chronic ulcer and chronic sinus tract; scar; and preexisting chronic dermatoses.6The most common cause of cSCC is UV exposure, with the majority of risk linked to ultraviolet B (290-320 nm) and additional risk from ultraviolet A (320-400 nm).6-8
Individuals with light skin that burns with increasing UV exposure have the highest risk for developing skin cancer.9Individuals with a history of sun exposure throughout childhood, especially those with a history of sunburn, may have the highest risk. The risk for cSCC is approximately 3 times higher in individuals born in areas that receive high amounts of UV radiation compared with those who move to sun-exposed locales in adulthood. Additionally, the risk for cSCC is up to 5 times higher in individuals with light skin color, blue or hazel eyes, and red or blonde hair compared with those who have darker skin, eyes, and hair.10 Increased risk has also been established in individuals with freckling, solar elastosis, or facial telangiectasia.10
Immunosuppression also contributes to the development of cSCC, particularly in the case of organ transplant recipients. Organ transplant recipients have approximately twice the overall risk for cancer, as compared with the general population.11In a nationwide study using Swedish registries, the risk for cSCC was increased 198-fold among heart and lung transplant recipients, 121-fold among kidney transplant recipients, and 32-fold among liver transplant recipients.12
Actinic keratosis (AK) is the most common precursor of cSCC. AKs are typically 2 mm to 6 mm in diameter and are scaly lesions that are often more easily felt than seen, as they may be pink, brown, or the same color as the skin.13Individuals with multiple keratoses have a lifetime risk of 0.1% to 14% for developing cSCC.14This cumulative risk depends on both the number of lesions and the length of time that the lesions persist, as well as other high-risk factors, such as prior radiation therapy, existing degree of photodamage, personal or familial history of skin cancer, Fitzpatrick skin type, and immunosuppression associated with disease or medications.15,16
Bowenoid papulosis and epidermodysplasia verruciformis are other precancerous lesions that may develop into cSCC.6,17Bowenoid papulosis is often associated with HPV types 16 and 18.6Patients often present with hyperpigmented papules that have histologic features in line with Bowen’s disease, which is a type of squamous cell carcinoma (SCC) in situ.18Epidermodysplasia verruciformis is a condition in which flat, widespread warts may degenerate into either carcinoma in situ or invasive SCC.19
If not adequately treated, SCC in situ may progress to invasive disease.6Common forms of SCC in situ include Bowen disease and Erythroplasia of Queyrat.20 Patients with Bowen disease often present with erythematous, scaly, or velvety plaques on sun-exposed areas that are well demarcated.21Occurring on the glans penis of uncircumcised men, Erythroplasia of Queyrat is less common and presents as smooth, red plaques.22
Presentation and Staging
The most common locations for invasive cSCCs are the head and neck, followed by the trunk.23cSCC often presents as a scaly, erythematous papule with or without ulceration and hemorrhagic crust on chronically sun-exposed skin in older patients.24Pronounced hyperkeratosis may be present, and large lesions may contain a central core of hard keratin and hemorrhagic debris. Most lesions are asymptomatic; however, some lesions may bleed or be tender to the touch. Additionally, patients may describe cSCC lesions as painful or itchy nonhealing wounds that may bleed with trauma.25
Upon presentation of cSCC, a physical examination and complete history should be taken, including information on sun exposure beginning in childhood, occupational exposure to UV light, prior radiation treatment, and possible causes of immunosuppression.13Patients with a history of skin cancer require screening to monitor for recurrence, persistence, or new lesions, because the risk for developing a second cSCC within 5 years following treatment of a first tumor is approximately 30%.26
For staging purposes, cSCC was grouped with various other cutaneous malignancies until the seventh edition of the American Joint Committee on Cancer (AJCC) staging manual was published in 2010 and cSCC was specifically addressed.27Several studies have evaluated the use of this staging system for cSCC, noting unsatisfactory prognostication in different staging groups.28Based on results from a retrospective cohort study demonstrating that the majority of poor outcomes occurred in AJCC T2 tumors, an alternative tumor staging system was developed (Brigham and Women’s Hospital [BWH] system).29The aim of the alternative staging system was to prognostically stratify the T2 group based on strongly predictive risk factors of at least 2 endpoints of interest. These risk factors include a tumor diameter of ≥2 cm, poorly differentiated histologic characteristics, perineural invasion, and tumor invasion beyond the subcutaneous fat.29
In this system, T1 tumors have no risk factors, T2a tumors have a single risk factor, T2b tumors have 2 to 3 risk factors, and T3 tumors have all 4 risk factors or bone invasion, coinciding with AJCC T3 and T4 cases.29Compared with the AJCC staging system, the BWH system does not address metastasis, nodal classifications, or advanced stage groups; however, the BWH system may provide improved prognostication for patients with localized cSCC.27These staging systems are compared in the TABLE.30
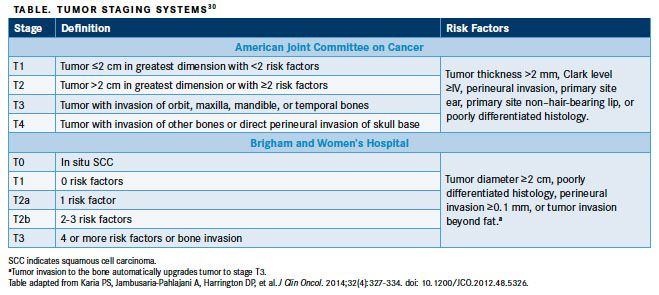
Patients with tumors classified as T2a using the alternative staging system comprised 26% of the aforementioned retrospective study cohort and experienced rare poor outcomes. Five-year cumulative incidence was 6% for local recurrence, 4% for nodal metastasis, 0% for death from cSCC, and 24% for all-cause death, which together account for 16% of all cSCCrelated events.29Patients with tumors classified as T2b using the alternative staging system comprised 19% of the cohort and experienced significantly higher incidences of poor outcomes. Five-year cumulative incidence was 18% for local recurrence, 37% for nodal metastasis, 20% for death from cSCC, and 47% for all-cause death. Together, alternative stage T2a tumors accounted for 64% of all cSCC-related events including 43% of local recurrences, 72% of nodal metastases, and 83% of deaths from cSCC.29
A study comparing the BWH staging system with the AJCC staging system validated the BWH system in a larger cohort of patients with cSCC.30Specifically, investigators reported that confidence intervals overlapped for AJCC T3 and T4 at all endpoints, demonstrating that these are not distinct stages, whereas BWH stages overlapped only slightly for the endpoint of overall death, demonstrating improved distinctiveness of all stages for the endpoints of local recurrence, nodal metastasis, and disease-specific death using BWH staging.30
According to the findings, 86% of poor outcomes were reported in stages T1 and T2 using AJCC staging; however, using BWH staging, 40% of poor outcomes occurred in stages T1 and T2a, demonstrating a greater degree of homogeneity.30The authors also assessed monotonicity, which occurs when higher proportions of poor outcomes occur at higher tumor stages.30In AJCC staging, 14% of poor outcomes occurred in stages T3 and T4.30In BWH staging, however, 60% of poor outcomes occurred in stages T2b and T3, including 70% of nodal metastases and 83% of disease-specific deaths, demonstrating greater monotonicity.30
Additional analyses evaluating upstaged and downstaged tumors using the BWH staging system compared with AJCC staging demonstrated that tumors downstaged by the BWH system to stage T1 and T2 have a low risk for poor outcomes, indicating appropriate downstaging.30Likewise, in the 86 cases upstaged from stage T1 and T2 in the AJCC staging system to stage T2b and T3 in the BWH staging system, there were 18 local recurrences, 18 nodal metastases, and 9 disease-specific deaths, indicating appropriate upstaging of these tumors based on poor outcomes.30
The National Comprehensive Cancer Network (NCCN) has also described an approach for stratifying cSCC using risk factors.31This risk stratification is intended to give healthcare providers practical clinical guidance for treating cSCC, rather than accurate prognostication. It is based on a combination of available evidence and expert opinion.27The NCCN risk stratification is based on the clinical factors of tumor location and size; border definition; recurrence status; immunosuppression; prior site of radiation or chronic inflammation; growth rate; and presence of neurologic symptoms. Pathologic factors, including degree of differentiation, histologic subtype, depth, and perineural, lymphatic, or vascular involvement, are also used to determine risk for cSCC.31
Evaluation and Diagnosis
Currently, no evidence-based, optimal biopsy technique exists for sampling potential cSCC lesions.27Recommended biopsy techniques include shave biopsy, punch biopsy, and excisional biopsy. The purpose of excisional biopsy is to determine and confirm a cSCC diagnosis, whereas the purpose of excision with margins is to remove the tumor. For any biopsy method, the specimen size and depth should be adequate for providing clinical information and pathology details to allow for an accurate diagnosis and therapy guidance.27
The selection of a specific biopsy technique is based on clinical characteristics of the tumor, patient-specific factors, patient preference, and physician judgment.27Clinical characteristics include morphology, expected histologic subtype and depth, natural history, and anatomic location. Patient-specific factors include bleeding and wound healing diatheses. Patient and physician preferences for minimizing biopsy-associated discomfort, trauma, infection risk, scar, or loss of function counterbalance the need to obtain information gleaned through biopsy.27These considerations are especially relevant on the head, neck, and other functional, sensory, or cosmetically sensitive sites. In the case of recurrent tumors, invasive tumors, or other aggressive tumor characteristics, additional biopsies or more extensive tissue resection may be required.27
A presumptive cSCC diagnosis is attained through a physician’s interpretation of clinical information (eg, appearance and morphology), anatomic location, and patientreported history.27Prior to treatment, the clinical diagnosis is routinely confirmed with biopsy findings. Along with biopsy tissue, additional information should be provided to the pathologist including patient age and gender; anatomic location; recurrence status; size of the lesion; immunosuppression status; and history related to radiation exposure, burns, and organ transplantation. The purpose of the pathology report is to convey an accurate diagnosis of the presence or absence of cSCC. In cases in which cSCC is detected, the degree of differentiation and any high- or low-risk features should be listed, including histologic subtype, depth of invasion, and the presence of perineural or lymphovascular invasion.27
A dermatologist or pathologist with experience interpreting cutaneous neoplasms should perform the pathologic evaluation of skin biopsy specimens. These clinicians can best interpret clinical findings and histologic features of tumors, thus providing the most accurate and precise biopsy diagnosis.27
Patients with large lesions (ie, ≥2 cm in diameter) have reported local recurrence rates of 15% and metastatic rates of 30%, which are 2 and 3 times the recurrence rates and metastatic rates, respectively, among those with of smaller lesions.32Patients with large tumors (ie, ≥2 cm in diameter) have 5-year cure rates of 58.3% to 74.8%%, whereas those with smaller tumors (ie, <2 cm in diameter) have 5-year cure rates of 83.5% to 98.1%.32
Other clinical features that are associated with local recurrence and metastasis include immunosuppression, poor histologic differentiation, and previous treatment.32The rates of both local recurrence and metastasis are 47% for tumors with evidence of perineural involvement. Additionally, patients who are immunosuppressed have a cSCC metastasis rate of 12.9%. cSCCs of the lip and ear are aggressive lesions, with rates of recurrence and metastasis ranging between 10% and 19%.32
Histologic features can also be used to predict recurrence and metastasis in patients with cSCCs. Predictive characteristics include involvement of the reticular dermis or subcutaneous fat, a depth of >4 mm, and penetration into muscle, cartilage, bone, or fascia.13Additionally, poorly differentiated cSCCs have been reported to have a recurrence rate of 28.6% and 5-year cure rates of 46.4% to 67.4%, whereas well-differentiated lesions have been reported to have a local recurrence rate of 13.6% and 5-year cure rates of 81.0% to 97.0%.32Conclusions cSCCs are the second most common form of skin cancer and are frequently caused by UV exposure. Although many tumors are localized, some may recur or metastasize. Early detection of precursor lesions, appropriate diagnosis, and staging of cSCCs is key to providing patients with appropriate prognostic information. Risk factors can be used for staging and predicting recurrence and metastasis.
References:
- Rogers HW, Weinstock MA, Feldman SR, Coldiron BM. Incidence estimate of nonmelanoma skin cancer (keratinocyte carcinomas) in the U.S. population, 2012. 2015;151(10):1081-1086. doi: 10.1001/jamadermatol.2015.1187.
- Schmults CD, Karia PS, Carter JB, et al. Factors predictive of recurrence and death from cutaneous squamous cell carcinoma: a 10-year, single-institution cohort study.JAMA Dermatol.2013;149(5):541-547. doi: 10.1001/jamadermatol.2013.2139.
- Lim HW, Collins SAB, Resneck JS Jr, et al. The burden of skin disease in the United States. J Am Acad Dermatol. 2017;76(5):958-972. doi: 10.1016/j.jaad.2016.12.043.
- Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. [published online September 12, 2018].CA Cancer J Clin.doi: 10.3322/caac.21492.
- Karia PS, Han J, Schmults CD. Cutaneous squamous cell carcinoma: estimated incidence of disease, nodal metastasis, and deaths from disease in the United States, 2012.J Am Acad Dermatol.2013;68(6):957-966. doi: 10.1016/j.jaad.2012.11.037.
- Johnson TM, Rowe DE, Nelson BR, Swanson NA. Squamous cell carcinoma of the skin (excluding lip and oral mucosa).J Am Acad Dermatol.1992;26(3 pt 2):467-484.
- Rundel RD. Promotional effects of ultraviolet radiation on human basal and squamous cell carcinoma.Photochem Photobiol.1983;38(5):569-575.
- Liang SB, Ohtsuki Y, Furihata M, et al. Sun-exposure- and aging-dependent p53 protein accumulation results in growth advantage for tumour cells in carcinogenesis of nonmelanocytic skin cancer.Virchows Arch.1999;434(3):193-199.
- Fears TR, Scotto J. Estimating increases in skin cancer morbidity due to increases in ultraviolet radiation exposure. Cancer Invest. 1983;1(2):119-126.
- English DR, Armstrong BK, Kricker A, et al. Demographic characteristics, pigmentary and cutaneous risk factors for squamous cell carcinoma of the skin: a casecontrol study.Int J Cancer.1998;76(5):628-634.
- Engels EA, Pfeiffer RM, Fraumeni JF Jr, et al. Spectrum of cancer risk among US solid organ transplant recipients.JAMA.2011;306(17):1891-1901. doi: 10.1001/ jama.2011.1592.
- Krynitz B, Edgren G, Lindelöf B, et al. Risk of skin cancer and other malignancies in kidney, liver, heart and lung transplant recipients 1970 to 2008--a Swedish populationbased study.Int J Cancer.2013;132(6):1429-1438. doi: 10.1002/ijc.27765.
- Alam M, Ratner D. Cutaneous squamous-cell carcinoma.N Engl J Med. 2001;344(13):975-983.
- Salasche S. Epidemiology of actinic keratoses and squamous cell carcinoma.J Am Acad Dermatol.2000;42(1 pt 2):4-7.
- Callen JP, Bickers DR, Moy RL. Actinic keratoses.J Am Acad Dermatol.1997;36(4):650-653.
- Glogau RG. The risk of progression to invasive disease.J Am Acad Dermatol.2000;42(1 pt 2):23-24.
- Kwa RE, Campana K, Moy RL. Biology of cutaneous squamous cell carcinoma.J Am Acad Dermatol.1992;26(1):1-26.
- Baron JM, Rübben A, Grussendorf-Conen EI. HPV 18-induced pigmented bowenoid papulosis of the neck.J Am Acad Dermatol.1999;40(4):633-634.
- Emsen IM, Kabaler IE. Epidermodysplasia verruciformis: an early and unusual presentation.Can J Plast Surg.2010;18(1):21-24.
- Guidelines of care for cutaneous squamous cell carcinoma. Committee on Guidelines of Care. Task Force on Cutaneous Squamous Cell Carcinoma.J Am Acad Dermatol.1993;28(4):628-631.
- Lee JW, Hur J, Yeo KY, Hu HJ, Kim JS. A case of pigmented Bowen’s disease.Ann Dermatol.2009;21(2):197-199. doi: 10.5021/ad.2009.21.2.197
- Weedon D. Tumors of the epidermis. Weedon D, ed. In: Weedon’s Skin Pathology Essentials, 3rd ed. London, UK: Churchill Livingstone; 2009:668-703.
- Gray DT, Suman VJ, Su WP, et al. Trends in the population-based incidence of squamous cell carcinoma of the skin first diagnosed between 1984 and 1992.Arch Dermatol.1997;133(6):735-740.
- Christensen SR. Recent advances in field cancerization and management of multiple cutaneous squamous cell carcinomas. F1000Res. 2018;7. doi: 10.12688/ f1000research.12837.1.
- Mills KC, Kwatra SG, Feneran AN, et al. Itch and pain in nonmelanoma skin cancer: pain as an important feature of cutaneous squamous cell carcinoma.Arch Dermatol.2012;148(12):1422-1423. doi: 10.1001/archdermatol.2012.3104.
- Frankel DH, Hanusa BH, Zitelli JA. New primary nonmelanoma skin cancer in patients with a history of squamous cell carcinoma of the skin. implications and recommendations for follow-up.J Am Acad Dermatol.1992;26(5 pt 1):720-726.
- Kim JYS, Kozlow JH, Mittal B, Moyer J, Olenecki T, Rodgers P; Work Group: Invited Reviewers. Guidelines of care for the management of cutaneous squamous cell carcinoma.J Am Acad Dermatol.2018;78(3):560-578. doi: 10.1016/j.jaad.2017.10.007.
- Clark JR, Rumcheva P, Veness MJ. Analysis and comparison of the 7th edition American Joint Committee on Cancer (AJCC) nodal staging system for metastatic cutaneous squamous cell carcinoma of the head and neck.Ann Surg Oncol.2012;19(13):4252-4258. doi: 10.1245/s10434-012-2504-2.
- Jambusaria-Pahlajani A, Kanetsky PA, Karia PS, et al. Evaluation of AJCC tumor staging for cutaneous squamous cell carcinoma and a proposed alternative tumor staging system.JAMA Dermatol.2013;149(4):402-410. doi: 10.1001/jamadermatol. 2013.2456.
- Karia PS, Jambusaria-Pahlajani A, Harrington DP, et al. Evaluation of American Joint Committee on Cancer, International Union Against Cancer, and Brigham and Women’s Hospital tumor staging for cutaneous squamous cell carcinoma.J Clin Oncol. 2014;32(4):327-334. doi: 10.1200/JCO.2012.48.5326.
- National Comprehensive Cancer Network Clinical Practice Guidelines in Oncology. Squamous Cell Skin Cancer. NCCN website. https://www.nccn.org/professionals/physician_ gls/pdf/squamous.pdf. Updated October 23, 2018. Accessed October 25, 2018.
- Rowe DE, Carroll RJ, Day CL Jr. Prognostic factors for local recurrence, metastasis, and survival rates in squamous cell carcinoma of the skin, ear, and lip. implications for treatment modality selection.J Am Acad Dermatol. 1992;26(6):976-990.
Biomarker Testing Paves the Way for Better Targeted Therapies in NSCLC
April 16th 2024At a live virtual event, Edward S. Kim, MD, MBA, discussed the evolving landscape of biomarker testing before making treatment decisions for patients with early-stage non–small cell lung cancer (NSCLC).
Read More
