Role of Immune Checkpoint Inhibitors in Non-Small Cell Lung Cancer Oncogenic Driver Mutations
Lung and bronchus cancer are the leading causes of cancer-related deaths in the United States and will be responsible for an estimated 154,050 American deaths in 2018. An estimated 234,030 new cases of lung and bronchus cancer will be diagnosed in 2018, which represents 13.5% of all new cancer diagnoses in the United States.
1Nonsmall cell lung cancer (NSCLC) is the most common type of lung cancer, comprising approximately 80% to 85% of lung cancer cases.2The 3 main types of NSCLC are large cell carcinoma (10%), squamous cell carcinoma (25%), and adenocarcinoma (40%), with numerous less frequent subtypes.2,3
Approximately 70% of patients with NSCLC are diagnosed with locally advanced or metastatic disease (stage IIIB/IV) at the time of diagnosis, with 40% of these newly diagnosed patients with NSCLC having stage IV disease.4,5The treatment of advanced, recurrent, and metastatic NSCLC is often associated with poor prognosis, and treatment is aimed to extend survival and provide symptom management.4,5
Recent advances in understanding of distinct tumor biology and immunology have allowed for the development of numerous targeted therapies and immune checkpoint inhibitors, which have improved overall survival (OS) in specific NSCLC populations. Molecular characterization has led to the definition of new subgroups of patients with distinct tumor biology who require specific treatments and strategies.
Prior to the introduction of molecular targeted therapy, first-line treatment for advanced-stage NSCLC was limited to systemic cytotoxic chemotherapy and radiation therapy.4,5Several factors continue to influence systemic anticancer therapy recommendations for patients with advanced NSCLC, including individual tumor histology, patient performance status, molecular and im-munologic tumor characteristics, and driver oncogene biomarker status. These are evaluated to guide decision making among cytotoxic chemotherapy, targeted agents, and immunotherapy. Approximately 83% to 85% of patients with stage IV NSCLC do not have oncogenic driver biomarker status and require first-line therapy recommendations distinct from those of the biomarker-defined populations.6
Updated Recommendations for Molecular Testing in NSCLC
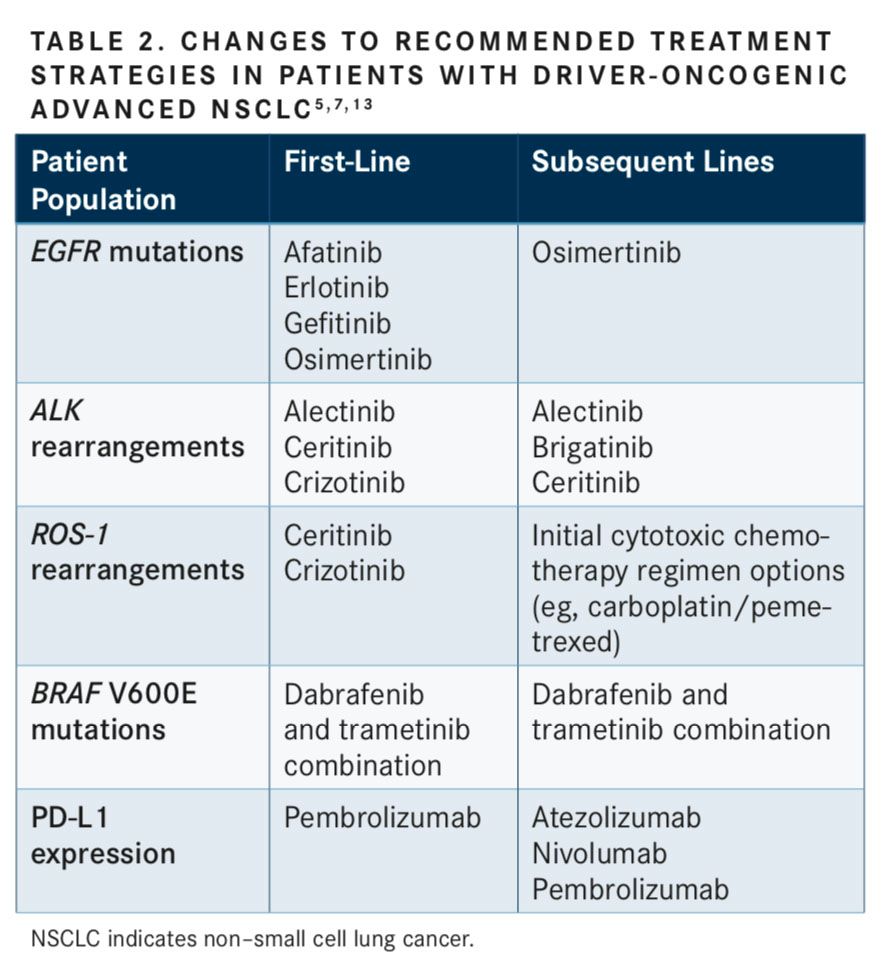
Guideline recommendations across several organizations indicate the need for molecular and biomarker testing of tumor samples as part of the routine diagnosis, prior to initiating therapy in the first-line setting, to guide treatment decisions. At minimum, patients must be tested for the 4 oncogenic driversEGFR mutations, ALK rearrangements, ROS-1 gene rearrangements, andBRAFV600E point mutationsas well as PD-L1 tumor expression level.5,7
In an interview withTargeted Oncology, Justin F. Gainor, MD, an attending physician in the Thoracic Oncology Group at Massachusetts General Hospital, emphasized the importance of genetic testing in NSCLC for appropriate treatment selection, “Over the past 15 years, there have been 2 major new paradigms that have transformed the management of nonsmall cell lung cancer: targeted therapy and immunotherapy. I can say that today, they still have very important roles in the management of our patients. Each has been guided by a different biomarker, and the importance of those biomarkers, I would say, are still very important today. So, patients with newly diagnosed advanced non–small cell lung cancer should still have comprehensive genetic profiling as well as PD-L1 expression scores.”
In an interview withTargeted Oncology, Alexander Drilon, MD, the clinical director of the Early Drug Development Service at Memorial Sloan Kettering Cancer Center, described the current landscape of genetic testing, “Paying attention to selecting the most appropriate test for profiling patients’ tumors on a molecular level is critical. [We have] really moved beyond single-gene testing that we used to do in the early 2000s when we knew about fewer genes likeEGFR, for example...and then later, theALKrearrangement. Now we have a whole slew of other potential drivers like ROS-1 and rearranged during transfection (RET) fusions, MET exon 14 alterations,BRAFV600E, andHER2. [It is] important to choose a test [that is] comprehensive and able to detect all of these alterations in patients who have nonsmall cell lung cancers. My personal preference is [to not] practice single- gene testing anymore and to go for a multiplex comprehensive approach.”
In the United States, approximately 20% of all NSCLC adenocarcinomas are characterized by the oncogenicEGFRmutations (10% to 15%), ALK rearrangements (3%- 7%),BRAFmutations (2%-5%), and ROS-1 rearrangements (0.5%-2%) that drive cancer growth.6,8-12As described in TABLE 1, these biomarkers can be DNA- or protein-based, requiring detection through sequencing and fluorescence in situ hybridization of immunohistochemistry (IHC), respectively.5,7,13Other driver oncogenes can be altered in NSCLC, includingKRAS,HER2,RETfusions, andMETexon 14 alterations, and additional genotypes contribute to 50% of NSCLC diagnoses.6,14Tyrosine kinase inhibitors (TKIs) are recommended in the first-line and subsequent treatment settings for patients with advanced nonsquamous NSCLC harboring oncogenic driver alterations, includingROS-1,BRAFV600E,EGFR, andALK.5
Patients with metastatic NSCLC with negative or unknown test results for oncogenic biomarkers are recommended to undergo IHC testing for PD-L1 expression prior to first-line treatment.5A positive PD-L1 biomarker expression level of greater than 50% expression is used to direct initial therapy. The FDA-approved checkpoint inhibitor pembrolizumab is indicated in the first-line setting, and nivolumab, atezolizumab, and pembrolizumab are indicated for use in the second-line setting following progression with first-line platinum-based chemotherapy for patients with metastatic, nonsquamous NSCLC with high PD-L1 expression (tumor proportion score [TPS] at least 50%) and without known oncogenic driver mutations.15-17Importantly, even in tumors with high PD-L1 expression, immunotherapy is not the standard of care for patients with mutations or rearrangements inEGFR,ALK,ROS-1, orBRAFin any line of therapy.
Predictive Biomarkers
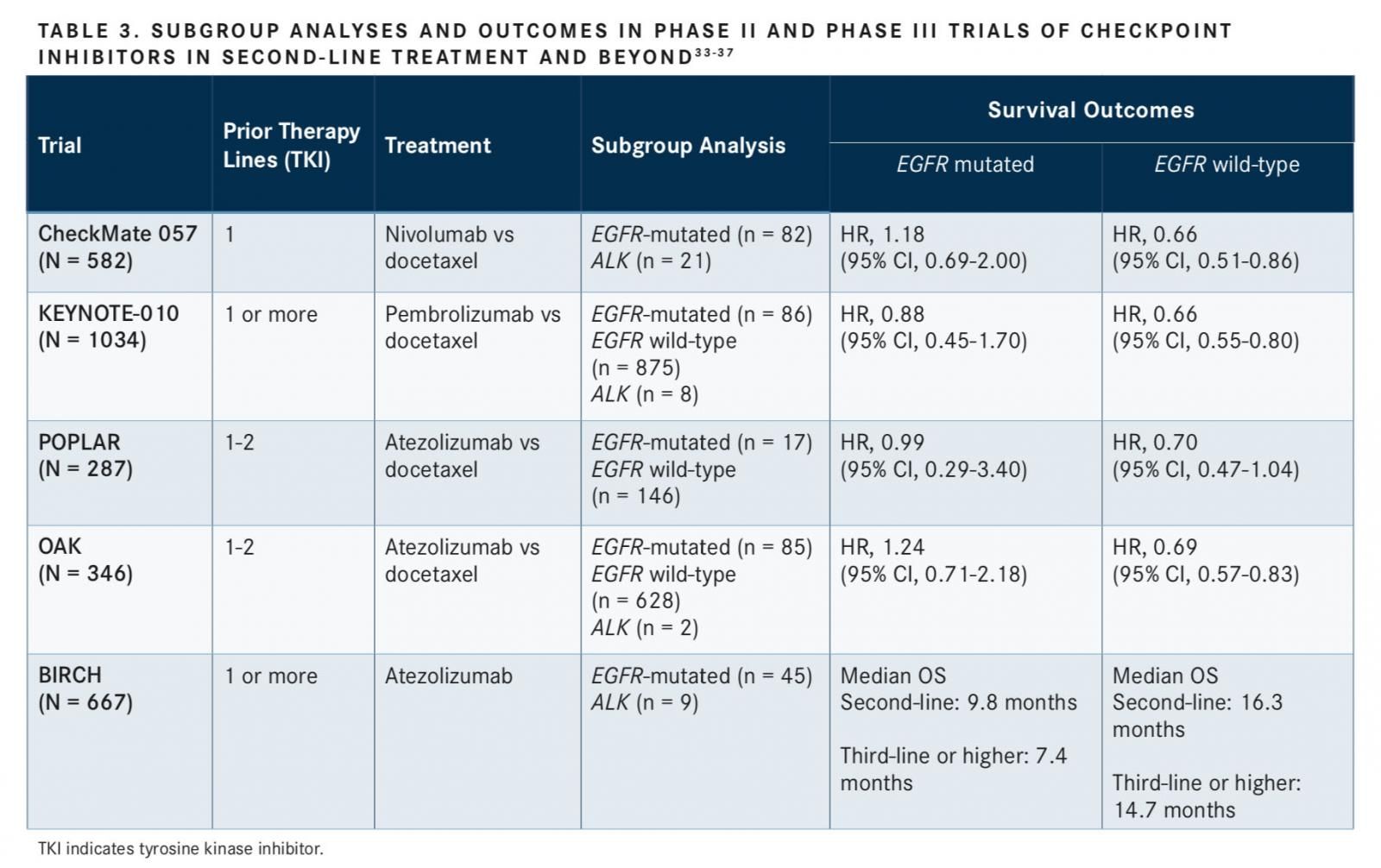
ROS-1,BRAFV600E,EGFR, andALKmolecular genetic abnormalities serve as predictive biomarkers of therapeutic response and are used to guide appropriate TKI treatment choice and subsequent guideline-recommended regimens (TABLE 2).5,7,13Patients across each biomarker-defined subpopulation may respond differently to first-line TKI treatment due to tumor heterogeneity. These genetic abnormalities can predict drug sensitivity as well as primary or acquired resistance to TKIs, which can guide selection of optimal second-line and subsequent therapy. For this reason, it is important to highlight the unique differences and heterogeneity in biomarker-defined patient populations.
Activating or sensitizingEGFRmutations are predictive of response; the most common mutations are deletions in exon 19 and the L858R point mutation in exon 21, both of which result in activation of the tyrosine kinase domain and are sensitive to the FDA-approved EGFR TKIs (gefitinib, erlotinib, and afatinib) for first-line treatment in patients with advancedEGFR-mutant NSCLC. Despite an initial response to TKIs, most of these patients will become resistant and experience disease progression after first-line TKI therapy.5,14SensitizingALKgene rearrangements are responsive to first-line pharmacological inhibition ofALKby TKIs such as alectinib, crizotinib, and ceritinib.5While crizotinib is highly active, most patients treated with crizotinib develop resistance.18,19
Patients withALK-positive NSCLC treated with anALKinhibitor in the first line can still respond to anotherALKinhibitor in the second and third lines. Brigatinib, alectinib, or ceritinib can be used in subsequent lines following progression after crizotinib treatment.18In a phase I study of 42 patients with crizotinib-resistant ALK-positive NSCLC, 31 achieved an objective response (OR) to treatment with brigatinib (objective response rate [ORR], 74%). The 31 patients with an OR had a median progression-free survival (PFS) of 14.5 months and 1-year OS of 83%.20In the phase II, ongoing, open-label, randomized, multicenter, international ALTA (ALK in Lung Cancer Trial of AP26113) trial, investigators studied brigatinib in patients with crizotinib-refractory, advanced, or metastaticALK-positive NSCLC (N = 222). Two doses of brigatinib were examined in this study, and treatment with the higher dose of 180 mg daily demonstrated considerable efficacy. Patients treated with 180 mg achieved a confirmed ORR of 54%; their 1-year OS was 80%, and their 1-year PFS was 54%.21 Based on the findings in these trials, the FDA granted brigatinib an accelerated approval in April 2017 for the treatment of patients with metastaticALK-positive NSCLC that has progressed on or is intolerant of crizotinib. The optimal sequence of therapy with brigatinib in the first line or after second-generationALKinhibitors is being further explored. The phase III ALTA-1L trial is investigating brigatinib efficacy and safety in patients with ALK-positive metastatic NSCLC as a first-line treatment compared with crizotinib.22
ROS-1 rearrangements are more common in patients who are negative forEGFRmutations andALKgene arrangements. ROS-1 confers response with crizotinib treatment in the first line, such asALKrearrangements. Brigatinib has demonstrated activity againstROS-1andEGFRTKI-resistant mutations, including T790M resistance mutation.7,23In the first-line setting,BRAFV600E mutations represent the majority of activating BRAF mutations and are responsive to first-line combination treatment with trametinib and dabrafenib.8,24-29
At this time, no single molecular determinant of response to an immunotherapeutic agent has been identified.4,5,7,13Notably, PD-L1 is not an optimal biomarker; the level of expression is variable. Despite PD-L1 expression in a subset ofEGFR-mutant andALK-positive NSCLCs, expression has been demonstrated to be highly dynamic, and expression levels change over time and in response to treatments.19The diagnostic PD-L1 IHC companion as- says are different with each agent, and some agents consider PD-L1 expression on both tumor cells and tumor- infiltrating immune cells when defining PD-L1 positivity.30Furthermore, the expression level of PD-L1 is subjective across clinical trials, and developments are still needed to better understand how levels of PD-L1 expression correlate with response.30
Other biomarkers, such as mutational load and smok- ing exposure, may be important potential determinants of response to PD-1 or PD-L1 inhibitors.31Drilon explained, “Tumor mutational burden [TMB], on a very simplistic level, is a measure of how complex a cancer is, [or] the number of mutations that occur within [a] tumor. We found that depending on the assay that [is used], you can measure tumor mutational burden by counting the number of non-synonymous mutations. If you have many more mutations, that counts as a high score versus if you have much fewer, that would be an intermediate or a lower score.” NSCLC with higher mutational load was associated with higher rates of durable response, as seen in patients with squamous NSCLC with smoking history.32As many targetable driver aberrations in adenocarcinomas, such asEGFRmutations andALKrearrangements, are not associated with smoking, these tumors are less likely to have DNA heterogeneity and subsequently, less neoantigen presentation conferring lack of response to immunotherapy.5
Guideline-Recommended Treatment Selection
A continuously evolving area of therapeutic development and recommendation is in the biomarker-defined population. For patients who experience disease progres- sion after second-line therapy, there is a need for widely applicable treatment approaches to extend survival,
minimize toxicity, and improve quality of life. Immunotherapy has expanded the range of treatment options for a distinct population of patients with advanced NSCLC who have disease progression following cytotoxic therapy. It has demonstrated consistent durable responses and long-term improvements in OS in comparison with single-agent docetaxel delivered in the second-line setting. Currently, treatment guidelines have removed recommendations supporting the use of any immune checkpoint inhibitors in NSCLC populations withALKrearrangements,ROS-1rearrangements,BRAFV600E mutations, or sensitizingEGFRmutations in those who have progressed on available TKIs who are candidates for subsequent treat- ment.7 NSCLC treatment guidelines recommend TKIs for these patient populations with progressive disease.7This change in recommendation was based on the emerging understanding of the role of checkpoint inhibitors and the growing body of clinical evidence that demonstrates that the subgroups of patients with classic oncogenic driving mutations do not achieve survival benefit or high rate of response in the second-line and subsequent settings with PD-1 or PD-L1 agents after progression on prior TKI therapy (TABLE 3).33-37Further analysis in the predictors of efficacy of antiPD-1 or anti–PD-L1 immunotherapy treatment in subgroups of patients with driver mutations is needed.
Emerging Clinical Evidence of PD-1/PD-L1 Treatment in Patients With Driver-Oncogenic NSCLC
Pivotal Immunotherapy Trials With Driver-Mutated Subgroup Analyses
CheckMate 057 Trial
Evidence from the CheckMate 057 trial demonstrated that the subgroup of patients withEGFR-mutated NSCLC who had disease progression and had received prior TKI or were re- ceiving an additional line of TKI are unlikely to benefit from nivolumab in the second-line or third-line setting, which con- tributed to the removal of immunotherapy from the guidelines in this population.7,33Nivolumab improved survival outcomes in the total population of patients with nonsquamous advanced NSCLC (stage IIIb or IV) compared with docetaxel.33In the subgroup analysis of theEGFR-mutated population (n = 82) in the CheckMate 057 trial, OS favored docetaxel compared with nivolumab (HR, 1.18; 95% CI, 0.69-2.00). PFS also favored docetaxel treatment in theEGFR-mutated population (HR, 1.46; 95% CI, 0.90-2.37). No data on sur- vival outcomes were reported for the subgroup withALKrearrangements (n = 21). A similar lack of benefit was observed for the subgroup of patients who never smoked. As discussed previously in this article, low levels of mutational load in neversmokers, as seen inEGFR- andALK- positive patients, may confer diminished sensitivity to immune checkpoint inhibitors.33
KEYNOTE-010 Trial
The phase II/III KEYNOTE-010 trial compared the efficacy and safety of pembrolizumab with docetaxel in patients with at least 1% PD-L1expressing advanced NSCLC in the second-line setting and beyond (N = 1034).34Patients with anEGFR-sensitizing mutation (n = 86) or anALKrearrangement (n = 8) who experienced disease progression following treatment with an appropriate TKI were also enrolled.34In theEGFRstatus subgroup analysis of the KEYNOTE-010 trial, docetaxel treatment was associated with favorable survival benefits compared with pembrolizumab, as evidenced by superior OS (HR, 0.88; 95% CI, 0.45-1.70) and PFS (HR, 1.79; 95% CI, 0.94-3.42). In contrast, pembrolizumab treatment reduced the risk of death and extended survival in the wild-typeEGFRsubgroups for both OS (HR, 0.66; 95% CI, 0.55-0.80) and PFS (HR, 0.83; 95% CI, 0.71- 0.98) compared with docetaxel, which was consistent with results in the overall treatment population.34
OAK and BIRCH Trials
Similar to previous trials with the immune checkpoint inhibitors nivolumab and pembrolizumab, the subgroup analyses of the OAK clinical trial demonstrated that atezolizumab did not exhibit favorable survival benefits as monotherapy in the second-line setting and beyond (progression with previous TKI) compared with docetaxel among patients with locally advanced or metastatic NSCLC withEGFRtumor aberrations, regardless of PD-L1 expression. In the subpopulation of patients withEGFR-mutated NSCLC (n = 85), docetaxel treatment improved median OS compared with atezolizumab (16.2 months vs 10.5 months; HR, 1.24; 95% CI, 0.71-2.18). However, atezolizumab increased median OS compared with docetaxel in theEGFRwild-type population (15.3 months vs 9.5 months; HR 0.69; 95% CI, 0.57-0.83). No data were reported for the 2 patients enrolled withALK-positive disease.36The disadvantage of atezolizumab in theEGFR-mutated population compared with the wild-typeEGFRpopulation added clinical evidence sup- porting the decision to remove all checkpoint inhibitors from the current treatment guidelines for patients with driver-mutated NSCLC.7,17,36,37
In the phase II BIRCH trial, patients withEGFR-mutated (n = 45) andALK-positive disease (n = 9) with PD-L1 expression in tumors and tumor-infiltrating lymphocytes had minimal responses to atezolizumab across the first-line and subsequent lines of therapy.17,35,37No data were reported for theALKsubpopulation. The ORRs to atezolizumab treatment in theEGFR-mutated cohort compared with theEGFRwild-type cohort were 23% and 19%, respectively, in the first-line setting (no prior chemotherapy for advanced NSCLC); 0% vs 21%, respectively, in the second line (progression during or following no more than 1 prior platinum-based regimen); and 7% vs 18%, respectively, in the third-line (progression during or following at least 2 prior chemotherapy regimens for advanced disease). Notably, median OS was substantially shorter in theEGFR-mutated cohort compared with theEGFRwild-type cohort treated with atezolizumab in the second line (6.8 months vs 16.3 months) and in the third line or beyond (3.4 months vs 14.7 months). Median PFS in the EGFR wild-type cohort was ap- proximately 2-fold greater than that observed in theEGFR-mutated cohort in the second and third lines.37
Response to Immune Checkpoint Inhibitors in EGFR-Mutant and ALK-Positive NSCLC
A retrospective analysis evaluated patients with EGFR mutations (n = 22) and ALK rearrangements (n = 6) and a matched comparator population (n = 30) ofEGFRwild-type andALK-negative patients treated with PD-1 or PD-L1 inhibitors at a single institution between 2011 to 2016. The majority of patients (82%) had disease progression following treatment with TKIs; 11% of patients were TKI-resistant and treated with a TKI in combination with a PD-1 inhibitor. Significantly more patients with wild-type disease achieved an OR compared with patients withEGFRmutations orALKrearrangements treated with PD-1 or PD-L1 inhibitors (23.3% vs 3.6%; P = .053). Only 1 patient withEGFRmutations orALKrearrangements achieved an objective response (OR) to pembrolizumab, which was not confirmed, but all ORs were confirmed in the wild-type population. Additionally, the median PFS in patients treated with PD-1 or PD-L1 inhibitors was significantly shorter inEGFR-mutant orALK-positive disease compared with nononcogenic driver-mutated disease (2.07 months vs 2.58 months; HR, 0.52; P = .018).19Notably, the ORRs observed in this single center study were similar to those acrossEGFRsubgroups in clinical trials (CheckMate 057 and KEYNOTE-010). In both studies, the PD-1 inhibitors produced response and survival benefits compared with docetaxel in the overall intention-to-treat populations, but inEGFR-mutant subgroup analyses, no survival benefit was observed.
ATLANTIC Trial
The phase II ATLANTIC trial was the first prospective evaluation of an immune checkpoint inhibitor in a dedicated cohort of patients withEGFR-mutated and ALK-translocated disease (n = 111). Durvalumab was evaluated in the third- line setting in patients with locally advanced or metastatic NSCLC, and patients were stratified based on tumor cell PD-L1 expression (high PD-L1 expression [≥25% or ≥90%] or low [<25%]).38 The ATLANTIC trial revealed valuable insights into the activity of durvalumab in driver-mutated NSCLC; EGFR-mutated and ALK-translocated molecular subpopulations have not only distinct tumor biology but also immunobiology.
PD-L1 expression alone was not predictive of efficacy in patients with mutated disease. Importantly, all ORs achieved in the mutated population were in EGFR-mutated patients, and no ORs were achieved by the ALK-positive subpopulation.EGFRandALKwild-type tumors had greater response to durvalumab, consistent with other PD-1 and PD-L1 inhibitors, than did mutated tumors. The ORR increased with the level of PD-L1 expression: the ORR was 12.2% inEGFR- orALK-positive NSCLC and high PD-L1 expression, and the ORR was 16.4% and 30.9% in the wild-type cohort with high PD-L1 expression of ≥25% and ≥90%, respectively.38In theEGFR-mutated NSCLC cohort, the ORR in patients with a high PD-L1 expression was 12.2% compared with 3.6% in patients with low PD-L1 expression. Only 1 EGFR-positive patient with low PD-L1 expression achieved an OR. These results suggest that immune checkpoint inhibitors are unlikely to induce respons- es in heavily pretreated EGFR-mutated patients. Additionally, regardless of PD-L1 positivity, this population has lower re- sponse to immunotherapy than wild-type populations.38
Gainor commented on the ATLANTIC trial, "I think the best prospective data with single-agent checkpoint inhibitors came from the ATLANTIC study. Notably, even when that study tried to enrich for responders by focusing on PD-L1 expressionin here, it was 25% or greater—the response rate amongEGFRpatients with that degree of PD-L1 expression was only 12%. And there were no responses observed in theALKsubgroup, despite having high PD-L1 expression. And I think this speaks to a point that PD-L1 expression is a less reliable predictive biomarker in patients withEGFR- andALK-positive disease. This is for several reasons, [the] first [being] that these tend to be low mutation tumors.EGFRandALKtend to occur in never- or light-smokers, and so we would expect them to have a low TMB."
Pooled Survival Outcomes From Key Immunotherapy Clinical Trials
Notably, patients with oncogenic driver mutations were frequently excluded from key clinical studies of PD-1 and PD-L1 agents. In this way, several meta-analyses have pooled subgroup analyses of patients with previously treatedEGFR-mutant advanced NSCLC across key clinical trials. These meta-analyses demonstrated that while the PD-1/PD-L1 inhibitors nivolumab, pembrolizumab, and atezolizumab improved OS and PFS in the wild-type EGFR population compared with docetaxel, no response or survival benefits were achieved in theEGFRsubgroup populations.
New Frontiers: Combination Chemotherapy Checkpoint Inhibitor Treatment in Driver-Oncogenic NSCLC
The role of checkpoint inhibitors in EGFR-mutated advanced NSCLC was investigated in a meta-analysis across 3 randomized clinical trials (CheckMate 057, KEYNOTE-010, POPLAR). Treatment with immune checkpoint inhibitors, including nivolumab (n = 292), pembrolizumab (n = 691), and atezolizumab (n = 144), was compared with docetaxel (n = 776) in the second-line or subsequent therapy setting (N = 1903). The intention-to-treat population achieved a 32% reduction in risk of death with immune checkpoint inhibitor therapy compared with docetaxel treatment (HR, 0.68; 95% CI, 0.61-0.77; P <.0001). Mirroring the OS benefit observed in the total treatment population, the wild-typeEGFRNSCLC subpopulation (n = 1362) achieved a 34% reduction in risk of death with treatment with immune checkpoint inhibitors (HR, 0.66; 95% CI, 0.58-0.76; P <.0001). Importantly, there was no reported OS advantage in theEGFR-mutated subpopulation (n = 186); immune checkpoint inhibitors were not superior to docetaxel for OS (HR, 1.05; 95% CI, 0.70-1.55; P = .81).39 A similar finding for OS in theEGFR-mutated NSCLC subpopulation was demonstrated in a second meta-analysis that expanded the data set to include the OAK trial. There was no OS benefit of PD-1 or PD-L1 inhibitor treatment compared with docetaxel in theEGFR-mutated NSCLC population (HR, 1.09; 95% CI, 0.84-1.41; P = .51). Furthermore, the PFS was significantly shorter in theEGFR-mutant subpopulation treated with PD-1 or PD-L1 inhibitors compared with docetaxel (HR, 1.44; 95% CI, 1.05-1.98; P = .02). In contrast, treatment with PD-1 or PD-L1 inhibitors compared with docetaxel in the wild-typeEGFRpopulation demonstrated benefits in OS (HR, 0.67; 95% CI, 0.61-0.76; P <.001) and had significantly longer PFS (HR, 0.86; 95% CI, 0.77-0.95; P = .004).40 The most recent me- ta-analysis included 5 key clinical trials (CheckMate 057, CheckMate 017, KEYNOTE-010, POPLAR, and OAK) and demonstrated that antiPD-1/PD-L1 treatment in previously treated, advanced NSCLC patients (N = 2752) was associated with a lower risk of death compared with docetaxel in EGFR wild-type tumors (HR, 0.67; 95% CI, 0.60-0.76), but not in EGFR-mutated tumors (HR, 1.12; 95% CI, 0.80-1.56).41
The Addition of Atezolizumab to Bevacizumab Plus Chemotherapy: IMpower150
The role of immune checkpoint inhibitor monotherapy in highly select patients with oncogene-driven tumors warrants additional studies to better define the patients who may derive benefit from the development of novel immune-based combinations. Combining different treat- ment modalities may potentially increase the clinical benefit of immunotherapy by increasing the immunogenicity of the tumor, bypassing immunological escape mechanisms at different levels of cellular benefit, and ad- dressing tumor heterogeneity. The potential benefits of im- munotherapy in ALK- or EGFR-positive NSCLC among patients with advanced refractory disease is currently being further explored in clinical trials evaluating combination immunotherapy and chemotherapy. The goal of these ongoing trials is to better understand immune-based treatment approaches and to better define and modulate the immune landscape ofALK- orEGFR-positive molecular subgroups of NSCLC.
Despite the available data that show poor efficacy of immune checkpoint inhibitor monotherapy in the treatment of advanced NSCLC patients withEGFRmutation orALKtranslocation, recent data from the IMpower 150 study indicate a potential role for the addition of a checkpoint inhibitor to combination platinum-doublet chemotherapy plus bevacizumab in these subgroups refractory to TKI treatment. Bevacizumab, an anti-VEGFagent, combined with chemotherapy is currently an approved frontline systemic treatment of metastatic non-squamous NSCLC.
In the phase III IMpower150 trial, combination treatment with atezolizumab in addition to bevacizumab-plus-chemotherapy (carboplatin and paclitaxel) resulted in a significant improvement in PFS and OS over bevacizumab-plus-chemotherapy alone, regardless of PD-L1 expres- sion andEGFRorALKgenetic alteration status. A total of 1202 patients with metastatic nonsquamous NSCLC were enrolled, including patients withEGFRandALKgenetic alterations (n = 108). These 108 patients had received treatment with at least 1 approved TKI, which had resulted in subsequent disease progression or unacceptable adverse events.42In theEGFRandALKwild-type population, PFS was significantly longer with the addition of atezolizumab to bevacizumab-plus-chemotherapy compared with bevacizumab-plus-chemotherapy alone (8.3 months vs 6.8 months; HR, 0.62; 95% CI, 0.52-0.74; P <.0001). Notably, this PFS benefit was also observed in the 14% of enrolled patients who hadEGFR- orALK-positive NSCLC (9.7 months vs 6.1 months; HR, 0.59; 95% CI, 0.37-0.94). The PFS benefit of atezolizumab combined with bevacizumab- plus-chemotherapy compared with bevacizumab-plus-chemotherapy alone was even more pronounced in those with actionableEGFRmutations (10.2 months vs 6.1 months).
The IMpower150 results are notable, as this chemotherapy-plus-immunotherapy combination was beneficial in the subgroups who have no phase III evidence supporting any benefit of PD-1 and PD-L1 inhibitor treatment as monotherapy or in combination with platinum-based regimens (that is, subgroups withEGFR- orALK-mutated NSCLC who have failed TKI treatments).42Gainor described the notable results of this trial, “It was a bit surprising to all of us to see that the benefits to adding atezolizumab to carboplatin/paclitaxel/bevacizumab even extended to theEGFR- andALK-positive subgroups compared with the control arm. The reason this was surprising is because in earli- er studies of just using single-agent checkpoint inhibitors, the benefits amongEGFR- andALK-positive patients were quite minimal with just single-agent checkpoint inhibitors. We saw response rates amongEGFRpatients were 10% or less, and in meta-analyses, there was no improvement in survival compared with single-agent docetaxel. And amongALK-positive patients, really the response rates have been 0 in the limited studies to date.”
Drilon commented on the future of combination treatment give the results from the IMpower150 trial, “The hope is that for [patients with] stage IV advanced nonsmall cell lung cancer [nonsquamous], the combination of chemo and immune therapy with bevacizumab might result in [a] response not just in tumors [that are] much more sensitive to immune therapy, but also in cancers that maybe fall below that cutoff like PD-L1.”
Gainor described the potential mechanism of the benefit of this combination regimen in the driver positive subgroup in the IMpower150, “One potential explanation is that chemotherapy may have led to tumor antigen release, and this may have spurred or resulted in increased sensitivity to the checkpoint inhibition. An alternative hypothesis is that there may have been an interplay between theVEGFinhibition with bevacizumab and PD-L1 inhibition with atezolizumab. That interplay may have been particularly important in patients withEGFR- andALK-positive lung cancer. Still, a third hypothesis is that there may have been something unusual about this particular subgroup of patients...It did seem like a disproportionate number of patients in had both [EGFR- andALK-positive lung cancer], which calls into question the molecular testing that was performed and the quality of testing. [This] raises the question [whether] all of these patients [were] trulyEGFRmutation-positive orALK-positive. Perhaps that may have explained some of the benefit.”
A Potential Role for Chemotherapy and Immunotherapy Combination in Oncogenic DriverPositive NSCLC
The results of the IMpower150 study highlight that there may be additional options for patients with ALK or EGFR genetic aberrations who are TKI-refractory. The role of chemotherapy in combination with immunotherapy will need to be further elucidated and determined for its place within the current treatment guideline.
Drilon explained the basis for this potential combination, “The rationale for adding chemotherapy to immune therapy is complex but can basically be broken down to the fact that chemotherapy drugs can, in a way, modulate the immune system. The purpose of adding chemotherapy to immune therapy is to hopefully boost the likelihood of immune therapy acting to augment the immune system. [This will make] tumors visible to the immune system and [it will attack] these cancers, getting rid of them.”
A new standard of care may emerge that includes chemotherapy combinations for those with driver-mutated disease, includingEGFRmutations andALKrearrangements. The role of chemotherapy in combination with checkpoint inhibitors may have potential utility in the second-line setting for patients who progress on TKI therapy in the front-line setting. However, the guideline-recommended strategy is the reintroduction of TKIs in later lines of therapy for patient populations with driver mutations who progress with a first-line TKI. As patients withEGFRmutations,ALKrearrangements,BRAFmutations, and ROS-1 rearrangements do respond to single-agent checkpoint inhibitors in the second line and beyond, appropriate use of chemotherapy in combination with immunotherapy will need to be understood with supporting evidence from clinical trials.
In an interview, Drilon stressed the need for better understanding of the appropriate sequencing and placement of this combination, “The question as to whether or not to use immune therapy for patients whose cancer is harboring an actionable driver is really a question of when to use immune therapy. So, it boils down to sequencing, essentially.” He continued to explain the current therapy placement of targeted therapies, “We know that for patients withEGFRmutations, recurrent gene rearrangements involvingALK,ROS-1, orRET;METexon 14 alterations;HER2mutations; orBRAFmutations that there are targeted therapies in development, several of which have very high response rates and good progression-free survival. So, when choosing therapies for patients, it’s all about the odds of responding to treatment. And you could argue that because for certain subsets of these driver-positive patients, likeEGFR,ALK,ROS-1, for example, where there are FDA-approved therapies andBRAF, that you should start with a targeted therapeutic because you’re seeing response rates in excess of 60% and very good progression-free survival.” Drilon continued, “If you were to think about a single-agent immune checkpoint inhibitor, this would be given relatively late in the course of therapy. I would argue that after targeted therapy, if you’re going to use chemotherapy alone, then you might use chemotherapy before going to single-agent immune checkpoint inhibitor.”
Gainor added his expert insight in treatment selection and sequencing, “We should really be prioritizing tar- geted therapies for those patients and really maximizing targeted therapies wherever possible for [them]. Unfortu- nately, we know that patients will eventually develop re- sistance to [targeted] therapy and, at some point, need to move on to other forms of therapy. I think the IMpower150 data tells us that one path forward may be exploring chemotherapy plus PD-1 combinations in those patients in- stead of just single-agent checkpoint inhibitors, which I think right now are not the best [way] forward for those particular patients.”
Drilon indicated the evolving treatment landscape and need for additional clinical evidence supporting the utlility of the combination in driver oncogenicpositive disease, “The question of whether or not to add immunotherapy to targeted therapy is an evolving question...while on a hypothetical level this might seem appealing, I wouldn’t personally do this as standard of care for patients. And this current point in time remains something that we explore on clinical trials. The bottom line is, right now, there are no strong data to support the use of a TKI together with immune therapy for any driver-positive subset.”
Conclusions
Patient populations with biomarker-defined NSCLC are highly heterogenous with distinct tumor biology, and there is a rapidly evolving conversation regarding the role of PD-1 or PD-L1 inhibitor monotherapy in subpopulations withEGFRmutations,ALKrearrangements,BRAFmutations, andROS-1rearrangements. Although PD-1 and PD-L1 agents do offer a new standard of care for a specific group of patients, checkpoint inhibitors are not an alternative therapy for NSCLC populations with the clas- sic driver oncogenic genetic alterations. Emerging clinical evidence demonstrates that PD-1 or PD-L1 inhibitors did not demonstrate superiority to docetaxel chemotherapy among patients withEGFR-mutant andALK-positive NSCLC in any line of therapy, supporting the removal of immunotherapy from the guidelines for treatment in this population after TKI failure. These agents should be evaluated further to determine their place in therapy. Prioritization in the development ofEGFR- andALK-targeted TKIs currently used in clinical practice are still the preferred options for reintroduction of targeted agents in later lines of therapy in these molecular NSCLC subgroups. Further exploration of the role of chemotherapy in combination with immunotherapy is needed to determine appropriate therapy selection (ie, when to reintroduce TKI vs chemo- therapy with PD-1 and PD-L1 inhibitors in subsequent lines). Importantly, the type of driver oncogene in NSCLC is distinct, and this tumor biology must be carefully considered with treatment selection across lines of therapy. Ultimately, each patient population must be managed with thoughtful understanding, as each subgroup will have different therapeutic responses to various therapeutic agents, given their unique molecular alterations.
References:
- Cancer stat facts: lung and bronchus cancer. National Cancer Institute / Surveillance, Epidemiology, and End Results website. seer.cancer.gov/statfacts/html/ lungb.html. Accessed June 7, 2018.
- What is non-small cell lung cancer? American Cancer Society website. cancer.org/ cancer/non-small-cell-lung-cancer/about/what-is-non-small-cell-lung-cancer.html. Updated May 16, 2016. Accessed August 1, 2018.
- Herbst RS, Heymach JV, Lippman SM. Lung cancer. N Engl J Med. 2008;359(13):1367-1380. doi: 10.1056/NEJMra0802714. 4. Novello S, Barlesi F, Califano R, et al; ESMO Guidelines Committee. Metastatic non-small-cell lung cancer: ESMO clinical practice guidelines for diagnosis, treat- ment and follow-up. Ann Oncol. 2016;27(suppl 5):v1-v27. doi: 10.1093/annonc/ mdw326.
- Novello S, Barlesi F, Califano R, et al; ESMO Guidelines Committee. Metastatic non-small-cell lung cancer: ESMO clinical practice guidelines for diagnosis, treat- ment and follow-up. Ann Oncol. 2016;27(suppl 5):v1-v27. doi: 10.1093/annonc/ mdw326.
- PDQ Adult Treatment Editorial Board. Non-small cell lung cancer treatment (PDQ®): health professional version. National Cancer Institute website. cancer.gov/ types/lung/hp/non-small-cell-lung-treatment-pdq. Published March 20, 2018. Ac- cessed August 7, 2018.
- Pao W, Girard N. New driver mutations in non-small-cell lung cancer. Lancet On- col. 2011;12(2):175-180. doi: 10.1016/S1470-2045(10)70087-5.
- NCCN Clinical Practice Guidelines in Oncology. Non-Small Cell Lung Cancer, version 4.2018. National Comprehensive Cancer Network website. nccn.org/ professionals/physician_gls/pdf/nscl.pdf. Published April 26, 2018. Accessed August 2, 2018.
- Farago AF, Azzoli CG. Beyond ALK and ROS-1: RET, NTRK, EGFR and BRAF gene rearrangements in non-small cell lung cancer. Transl Lung Cancer Res. 2017;6(5):550- 559. doi: 10.21037/tlcr.2017.08.02.
- Sharma SV, Bell DW, Settleman J, Haber DA. Epidermal growth factor receptor mutations in lung cancer. Nat Rev Cancer. 2007;7(3):169-181. doi: 10.1038/nrc2088.
- Dagogo-Jack I, Shaw AT. Crizotinib resistance: implications for therapeutic strat- egies. Ann Oncol. 2016;27(suppl 3):iii42-iii50. doi: 10.1093/annonc/mdw305.
- Patel JN, Ersek JL, Kim ES. Lung cancer biomarkers, targeted therapies and clinical assays. Transl Lung Cancer Res. 2015;4(5):503-514. doi: 10.3978/j.issn.2218- 6751.2015.06.02.
- Solomon BJ, Bauer TM, Felip E, et al. Safety and efficacy of lorlatinib (PF- 06463922) from the dose-escalation component of a study in patients with advanced ALK+ or ROS-1+ non-small cell lung cancer (NSCLC). J Clin Oncol. 2016;34(suppl 15):9009. doi: 10.1200/JCO.2016.34.15_suppl.9009.
- Lindeman NI, Cagle PT, Aisner DL, et al. Updated molecular testing guideline for the selection of lung cancer patients for treatment with targeted tyro- sine kinase inhibitors: guideline from the College of American Pathologists, the International Association for the Study of Lung Cancer, and the Association for Molecular Pathology. Arch Pathol Lab Med. 2018;142(3):321-346. doi: 10.5858/ arpa.2017-0388-CP.
- Riely GL. What, when, and how of biomarker testing in non-small cell lung cancer. J Natl Compr Canc Netw. 2017;15(5S):686-688. doi: 10.6004/jnccn.2017.0073.
- Keytruda [prescribing information]. Whitehouse Station, NJ: Merck & Co, Inc; 2018.
- Opdivo [prescribing information]. Princeton, NJ: Bristol-Myers Squibb Co; 2018.
- Tecentriq [prescribing information]. San Francisco, CA: Genentech, Inc; 2018.
- Mezquita L, Planchard D. The role of brigatinib in crizotinib-resistant non-small cell lung cancer. Cancer Manag Res. 2018;10:123-130. doi: 10.2147/ CMAR.S129963.
- Gainor JF, Shaw AT, Sequist LV, et al. EGFR mutations and ALK rearrangements are associated with low response rates to PD-1 pathway blockade in non-small cell lung cancer: a retrospective analysis. Clin Cancer Res. 2016;22(18):4585-4593. doi: 10.1158/1078-0432.CCR-15-3101.
- Gettinger SN, Bazhenova LA, Langer CJ, et al. Activity and safety of brigatinib in ALK-rearranged non-small-cell lung cancer and other malignancies: a single-arm, open-label, phase 1/2 trial. Lancet Oncol. 2016;17(12):1683-1696. doi: 10.1016/ S1470-2045(16)30392-8.
- Kim DW, Tiseo M, Ahn MJ, et al. Brigatinib in patients with crizotinib-refractory anaplastic lymphoma kinase-positive non-small-cell lung cancer: a randomized, multicenter phase II trial. J Clin Oncol. 2017;35(22):2490-2498. doi: 10.1200/ JCO.2016.71.5904.
- ALTA-1L study: a phase 3 study of brigatinib versus crizotinib in ALK-positive advanced non-small cell lung cancer patients (ALTA-1L). ClinicalTrials.gov website. clinicaltrials.gov/ct2/show/NCT02737501. Published April 14, 2016. Updated February 6. 2018. Accessed August 2, 2018.
- Camidge DR, Kim DW, Tiseo M, et al. Exploratory analysis of brigatinib activity in patients with anaplastic lymphoma kinase-positive non-small-cell lung cancer and brain metastases in two clinical trials. J Clin Oncol [published online May 16, 2018]. doi: 10.1200/JCO.2017.77.5841.
- Rossi A, Maione P, Santabarbara G, et al. The safety of second-line treatment options for non-small cell lung cancer. Expert Opin Drug Saf. 2017;16(4):471-479. doi: 10.1080/14740338.2017.1297795.
- Barlesi F, Mazieres J, Merlio JP, et al; Biomarkers France contributors. Routine molecular profiling of patients with advanced non-small-cell lung cancer: results of a 1-year nationwide programme of the French Cooperative Thoracic Intergroup (IFCT). Lancet. 2016;387(10026):1415-1426. doi: 10.1016/S0140-6736(16)00004-0.
- Tissot C, Couraud S, Tanguy R, et al. Clinical characteristics and outcome of patients with lung cancer harboring BRAF mutations. Lung Cancer. 2016;91:23-28. doi: 10.1016/j.lung- can.2015.11.006.
- Litvak AM, Paik PK, Woo KM, et al. Clinical characteristics and course of 63 patients with BRAF mutant lung cancers. J Thorac Oncol. 2014;9(11):1669-1674. doi: 10.1097/ JTO.0000000000000344.
- Cardarella S, Ogino A, Nishino M, et al. Clinical, pathologic, and biologic features associated with BRAF mutations in non-small cell lung cancer. Clin Cancer Res. 2013;19(16):4532-4540. doi: 10.1158/1078-0432.CCR-13-0657.
- Planchard D, Besse B, Groen HJM, et al. Dabrafenib plus trametinib in patients with previous- ly treated BRAF(V600E)-mutant metastatic non-small cell lung cancer: an open-label, multicen- tre phase 2 trial. Lancet Oncol. 2016;17(7):984-993. doi: 10.1016/S1470-2045(16)30146-2. 30. Teixidó C, Vilariño N, Reyes R, Reguart N. PD-L1 expression testing in non-small cell lung cancer. Ther Adv Med Oncol. 2018;10:1758835918763493. doi: 10.1177/1758835918763493.
- Teixidó C, Vilariño N, Reyes R, Reguart N. PD-L1 expression testing in non-small cell lung cancer. Ther Adv Med Oncol. 2018;10:1758835918763493. doi: 10.1177/1758835918763493.
- Rizvi NA, Hellmann MD, Snyder A et al. Cancer immunology. mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science. 2015;348(6230):124- 128. doi: 10.1126/science.aaa1348.
- Brahmer J, Reckamp KL, Baas P et al. Nivolumab versus docetaxel in advanced squa- mous-cell non-small-cell lung cancer. N Engl J Med. 2015;373(2):123-135. doi: 10.1056/NEJ- Moa1504627.
- Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab versus docetaxel in advanced nonsqua- mous non-small-cell lung cancer. N Engl J Med. 2015;373(17):1627-1639. doi: 10.1056/NEJ- Moa1507643.
- Herbst RS, Baas P, Kim DW, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet. 2016;387(10027):1540-1550. doi: 10.1016/S0140-6736(15)01281-7.
- Fehrenbacher L, Spira A, Ballinger M, et al; POPLAR Study Group. Atezolizumab versus docetaxel for patients with previously treated non-small-cell lung cancer (POPLAR): a multicen- tre, open-label, phase 2 randomised controlled trial. Lancet. 2016;387(10030):1837-1846. doi: 10.1016/S0140-6736(16)00587-0.
- Rittmeyer A, Barlesi F, Waterkamp D, et al; OAK Study Group. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, mul- ticentre randomised controlled trial. Lancet. 2017;389(10066):255-265. doi: 10.1016/S0140- 6736(16)32517-X.
- Peters S, Gettinger S, Johnson ML, et al. Phase II trial of atezolizumab as first-line or subsequent therapy for patients with programmed death-ligand 1-selected advanced non-small-cell lung cancer (BIRCH). J Clin Oncol. 2017;35(24):2781-2789. doi: 10.1200/JCO.2016.71.9476. 38. Garassino MC, Cho BC, Kim JH, et al; ATLANTIC Investigators. Durvalumab as third-line or later treatment for advanced non-small-cell lung cancer (ATLANTIC): an open-label, single-arm, phase 2 study. Lancet Oncol. 2018;19(4):521-536. doi: 10.1016/S1470-2045(18)30144-X.
- Garassino MC, Cho BC, Kim JH, et al; ATLANTIC Investigators. Durvalumab as third-line or later treatment for advanced non-small-cell lung cancer (ATLANTIC): an open-label, single-arm, phase 2 study. Lancet Oncol. 2018;19(4):521-536. doi: 10.1016/S1470-2045(18)30144-X.
- Lee CK, Man J, Lord S, et al. Checkpoint inhibitors in metastatic EGFR-mutated non- small cell lung cancera meta-analysis. J Thorac Oncol. 2017;12(2):403-407. doi: 10.1016/j. jtho.2016.10.007.
- Dong ZY, Zhang JT, Liu SY, et al. EGFR mutation correlates with uninflamed phenotype and weak immunogenicity, causing impaired response to PD-1 blockade in non-small cell lung can- cer. Oncoimmunology. 2017;6(11):e1356145. doi: 10.1080/2162402X.2017.1356145.
- Jiang Q, Xie M, He M, et al. Anti-PD-1/PD-L1 antibodies versus docetaxel in patients with previously treated non-small-cell lung cancer. Oncotarget. 2018;9(7):7672-7683. doi: 10.18632/oncotarget.23584.
- Socinski MA, Jotte RM, Cappuzzo F, et al; IMpower150 Study Group. Atezolizumab for first- line treatment of metastatic nonsquamous NSCLC. N Engl J Med. 2018;378(24):2288-2301. doi: 10.1056/NEJMoa1716948.
Multiple Resistance Mechanisms Thwart Efficacy for Third-Generation TKIs in NSCLC
April 7th 2024An important clinical strategy that focuses on improved frontline EGFR TKI regimens may help combat acquired EGFR TKI resistance and further streamline treatment options in subsequent lines, according to Balazs Halmos, MD.
Read More
