Emerging Treatment Strategies in MCL
The potential synergy of new agents with other treatment strategies, including immunotherapeutic and targeted approaches, is currently being investigated in various clinical trials, with the hope of identifying combinations that will lead to longer responses and improvements in duration of response for patients with MCL.
1New targeted agents in development for MCL include BTK inhibitors, BCL-2 inhibitors, and phosphoinositide 3-kinase (PI3K) inhibitors.2Emerging therapies also include novel immunotherapeutic regimens using immune checkpoint inhibitors and T-cellbased adoptive immunotherapy.3
According to David Tucker, MD, and Simon Rule, MD, “In addition to improvements in immunochemotherapy, a succession of new molecular targets and corresponding drugs has revolutionized MCL therapy. The best way to sequence and combine these agents with existing regimens and how to overcome the problem of drug resistance represent new challenges in this rapidly developing field.”4
The potential synergy of these new agents with other treatment strategies, including immunotherapeutic and targeted approaches, is currently being investigated in various clinical trials, with the hope of identifying combinations that will lead to longer responses and improvements in duration of response (DOR) for patients with MCL.
Ibrutinib Combinations
Ibrutinib is a potent orally bioavailable inhibitor of BTK that binds irreversibly to the cysteine residue (C481) at the phosphorylation site of BTK, leading to irreversible inactivation and disruption of the signaling pathway from the B-cell receptor (BCR) to the nucleus.4The BCR signaling pathway plays a crucial role in cell division, differentiation, homing, and survival.5The promising ef cacy and safety of single-agent ibrutinib demonstrated in the relapsed or refractory (R/R) setting has led to considerable interest in sequencing and combination regimens with other agents.4
The combination of ibrutinib and rituximab was evaluated in a phase II single-center open-label trial of 50 patients with R/R MCL.6Preliminary trial results show this combination to be well tolerated and highly effective in patients with Ki-67 ˂50% who achieved an objective response rate (ORR) of 100% and a complete remission (CR) rate of 54%. However, response was substantially reduced in patients with Ki-67 ˃50% who had an ORR of 50% and a CR rate of 8%. Atrial brillation (AF) was noted in 6 patients (12%) and was the only grade 3 adverse event (AE) occurring in more than 10%. Two grade 4 AEs were also reported (1 each of diarrhea and neutropenia).
Previous studies have demonstrated that ibrutinib monotherapy and combination therapy with lenalidomide plus rituximab have high activity in MCL.7,8Based on these results, the combination of ibrutinib, lenalidomide, and rituximab was explored in the phase II open-label PHILEMON trial of patients with R/R MCL.9A total of 50 patients were enrolled and received induction therapy with rituximab, ibrutinib, and lenalidomide for up to 12 cycles. Patients who had a CR, partial remission (PR), or stable disease entered a maintenance phase, with treatment consisting of ibrutinib and rituximab only. After a median follow-up of 17.8 months, the ORR was 76% and the CR rate was 56%. The most common grade ≥3 AEs included neutropenia (38%), infection (22%), and cutaneous toxicity (14%).
Palbociclib (Ibrance) is an oral, speci c CDK4/6 inhibitor that has demonstrated cytotoxicity against the mutated BTKC481S protein.10Prolonged early G1 cell-cycle arrest induced by palbociclib can overcome ibrutinib resistance in primary human samples and MCL cell lines with wild-type BTK.11Adding palbociclib to ibrutinib could help deepen the response seen with ibrutinib through dual CDK 4/6 and BTK inhibition in MCL.
Peter Martin, MD, and colleagues12conducted a phase I dose-escalation study to evaluate the safety and preliminary activity of palbociclib plus ibrutinib in patients with previously treated MCL. Phase I data suggest that this is an active combination in patients with MCL. Twelve of 18 patients (67%) responded to treatment and 8 (44%) achieved CR. After a median follow-up of 14 months, the rate of progression-free survival (PFS) at 1 year was 61%; only 1 patient had disease progression. Grade 3 cutaneous toxicity was observed at the highest dosage (ibrutinib 560 mg/palbociclib 125 mg); otherwise, grade 1/2 myelosuppression was the most common AE.
Ibrutinib and the BCL-2 inhibitor venetoclax have demonstrated synergy in cell lines and primary cells, and study findings have indicated the potential for combining these agents in the treatment of MCL.13,14The phase II open-label AIM study15 evaluated ibrutinib plus venetoclax in patients with R/R MCL. At 16 weeks, the ORR was 71% and the CR rate was 63%. After a median follow-up of 8.3 months, the PFS rate was 74% and the overall survival (OS) rate was 81%. The most common AEs were fatigue (71%), diarrhea (67%), nausea (50%), upper respiratory tract infection (38%), gastroesophageal re ux (33%), neutropenia (33%), cough (25%), and bruising (21%).
Acalabrutinib Combinations
Acalabrutinib is another oral BTK inhibitor, and it binds covalently to C481.4In evaluating pharmacokinetic properties of acalabrutinib in healthy adult patients with B-cell malignancies, rapid absorption and fast elimination was observed.
The phase III ACE-LY-309 study16of acalabrutinib in combination with rituximab versus ibrutinib versus acalabrutinib for the treatment of R/R MCL was halted prior to enrollment. The ongoing phase Ib ACE-LY-016 trial17is investigating the combination of acalabrutinib plus bendamustine (Treanda) and rituximab in patients with treatment-naïve and R/R MCL. The primary outcome measure is safety as determined by the number of participants with treatment-emergent AEs.
CAR T-Cell Therapy
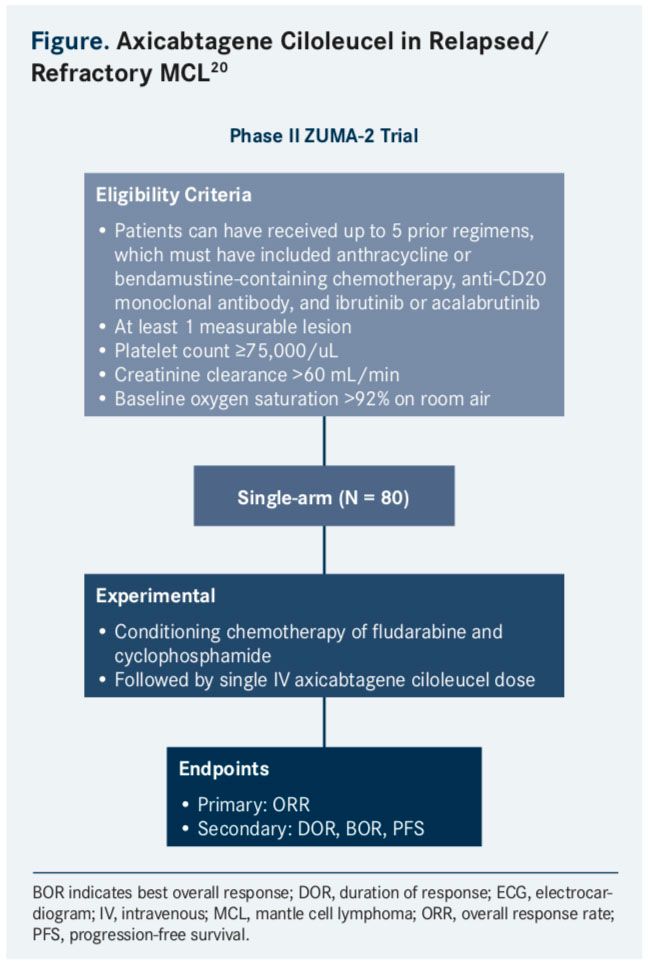
Ongoing studies are exploring anti-CD19 CAR T-cell therapy in patients with chemorefractory MCL.18CAR T-cell therapy involves the modication of T cells via lentiviral transduction to express specific CARs; in B-cell lymphomas, CD19 is a common target for T cells.3A phase I/II study is currently underway to investigate the safety and ef cacy of anti-CD19 CAR T-cell therapy in older patients with R/R MCL.19The phase II ZUMA-2 study is evaluating axicabtagene ciloleucel (KTE-C19; Yescarta) in patients with ibrutinib refractory MCL (Figure).20The estimated study completion date is July 2018.
According to Raphael Steiner, MD, and colleagues, “In order for CAR T-cell therapies to be more widely adopted in chemorefractory relapsed or refractory MCL, they will have to demonstrate an ability to safely induce responses in patients who would not be eligible for allogeneic stem cell transplant. The toxicities associated with CAR T cells at present may limit applicability to patients [with MCL] who are commonly older and have comorbid conditions.”1
Immune Checkpoint Inhibitors
Antibodies targeting programmed cell death-1 protein (PD-1) have been investigated in lymphoid malignancies with varying levels of activity and a favorable toxicity profile.21The first checkpoint inhibitor approved for lymphomas was nivolumab, a PD-1 inhibitor, which was approved in May 2016 for patients with R/R Hodgkin lymphoma. Currently, data on response to immune checkpoint inhibition and combinations with checkpoint blockade in MCL are limited. However, an ongoing phase I/ Ib trial is investigating the safety and maximum-tolerated dose (MTD) of the PD-1 inhibitor pembrolizumab (Keytruda) combined with ibrutinib in patients with non-Hodgkin lymphoma (NHL), including those with MCL.22
Venetoclax
Venetoclax (Venclexta) is an oral small molecule BCL-2 inhibitor that has been investigated for the treatment of NHL. BCL-2 is an antiapoptotic factor involved in the regulation of cyclin D1; targeting BCL-2 may also induce apoptosis.2
A phase I study investigated the safety, pharmacokinetics, and ef cacy of venetoclax in patients with NHL.23A total of 106 patients with R/R NHL, including 26 with diagnosed MCL, were enrolled in the study, which consisted of a dose-escalation stage and a safety expansion stage. Preliminary results show impressive single-agent activity in R/R MCL, with negligible toxicity, apart from risk of tumor lysis syndrome (TLS). An ORR of 75% (21/28) was observed among the patients with a diagnosis of MCL. The median DOR was 5.3 months (range, 0.2-46.0) for the total study population but was not reported speci cally for patients with MCL. The most common any-grade AEs were nausea (48%), diarrhea (45%), and fatigue (42%). The most common grade ≥3 AEs were anemia (15%), neutropenia (11%), and thrombocytopenia (9%). Laboratory TLS was reported in 3 patients, with no cases of clinical TLS observed.
In addition to the ibrutinib/venetoclax combination, the combination of venetoclax with bendamustine, rituximab, and ibrutinib is being explored in an ongoing phase I trial of patients with R/R MCL.24The primary outcome measures are MTD and safety.
Idelalisib
Idelalisib is an oral selective inhibitor of the PI3Kδ isoform. PI3Kδ plays a pivotal role in the BCR signaling pathway and is hyperactivated in B-cell malignancies, including MCL.4Combination regimens are being explored with idelalisib in early-phase studies, as well.
The A051201 study25,26investigated lenalidomide with or without idelalisib in patients with R/R MCL. A051201, which included a phase I dose-escalation study followed by a phase II study, was originally designed to capitalize on the clinical synergy of lenalidomide and rituximab observed in previous trials by adding the highly speci c PI3Kδ inhibitor, idelalisib. Due to excessive toxicity, the trial was amended to remove the rituximab treatment arm. The study was closed in May 2017.
An ongoing phase Ib open-label study is examining the safety and ef cacy of the BCL-2 inhibitor S55746 in combination with idelalisib in patients with R/R follicular lymphoma and those with MCL. This dose-escalation and dose-expansion study is the first to study a clinical combination of S55746 with another targeted agent.27
Rituximab After Stem Cell Transplantation in MCL
Maintenance treatment with rituximab following autologous stem cell transplantation (ASCT) has been shown to prolong event-free survival (EFS), PFS, and OS in patients with MCL who are ˃66 years. In the phase III LyMa trial,28299 patients with MCL received 4 courses of R-DHAP (rituximab, dexamethasone, cytarabine, cisplatin) followed by R-BEAM (rituximab, carmustine, etoposide, cytarabine, melphalan)/ASCT; patients who did not achieve at least a PR after R-DHAP could receive 4 additional courses of R-CHOP (rituximab, cyclophosphamide, hydroxydaunomycin [doxorubicin], vincristine, and prednisone) to facilitate ASCT. The intent-to-treat population consisted of 240 responders who were randomly assigned to receive 3 years of rituximab maintenance or observation. After a median follow-up of 50.2 months, the rate of EFS at 4 years was 79% in the rituximab group versus 61% in the observation group (P = .001). The rate of PFS at 4 years was 83% in the rituximab group versus 64% in the observation group (P <.001), and OS was 89% versus 80%, respectively (P = .04).
Maintenance Rituximab
The use of ASCT consolidation in conjunction with maintenance rituximab is also being evaluated by the ECOG-ACRIN Cancer Research Group in an phase III open-label trial of patients with MCL experiencing their first CR.29The study will compare OS in patients with MCL who are minimal residual diseasenegative following induction chemotherapy (investigator’s choice) to either ASCT followed by maintenance rituximab or to maintenance rituximab alone. The findings of the study will help to elucidate the relative value of each component of this treatment paradigm.30
References:
- Steiner RE, Romaguera J, Wang M. Current trials for frontline therapy of mantle cell lymphoma. J Hematol Oncol. 2018(11);11:13. doi: 10.1186/s13045-018-0556-x.
- Arora PC, Portell CA. Novel therapies for relapsed/refractory mantle cell lymphoma. Best Pract Res Clin Haematol. 2018;31(1):105-113. doi: 10.1016/j. beha.2017.10.010.
- Robak T. Novel therapies under investigation for mantle cell lymphoma. Expert Opin Investig Drugs. 2016;25(4):375-380. doi: 10.1517/13543784.2016.1152259.
- Barf T, Covey T, Izumi R, et al. Acalabrutinib (ACP-196): a covalent Bruton tyrosine kinase inhibitor with a differentiated selectivity and in vivo potency pro le. J Pharmacol Exp Ther. 2017;363:240-252. doi: 10.1124/jpet.117.242909.
- Inamdar AA, Goy A, Ayoub NM, et al. Mantle cell lymphoma in the era of precision medicine-diagnosis, biomarkers and therapeutic agents. Oncotarget. 2016;7(30):48692-48731. doi: 10.18632/oncotarget.8961.
- Wang ML, Lee H, Chuang H, et al. Ibrutinib in combination with rituximab in relapsed or refractory mantle cell lymphoma: a single-centre, open-label, phase 2 trial. Lancet Oncol. 2016;17(1):48-56. doi: 10.1016/S1470-2045(15)00438-6.
- Wang ML, Rule S, Martin P, et al. Targeting BTK with ibrutinib in relapsed or refractory mantle-cell lymphoma. N Engl J Med. 2013;369(6):507-516. doi: 10.1056/NEJMoa1306220.
- Wang M, Fayad L, Wagner-Bartak N, et al. Lenalidomide in combination with rituximab for patients with relapsed or refractory mantle-cell lymphoma: a phase 1/2 clinical trial. Lancet Oncol. 2012;13(7):716-723. doi: 10.1016/S1470-2045(12)70200-0.
- Jerkeman M, Eskelund CW, Hutchings M, et al. Ibrutinib, lenalidomide, and rituximab in relapsed or refractory mantle cell lymphoma (PHILEMON): a multicentre, open-label, single-arm, phase 2 trial [published online January 29, 2018]. Lancet Haematol. doi: 10.1016/S2352-3026(18)30018-8 .
- Pant MK, Alshenawy W, Alrajjal A, Alrobeh H, Tabbara IA. Mantle cell lymphoma: current concepts. J Hematol Thrombo Dis. 2015;3:215. omicsonline. org/open-access/mantle-cell-lymphoma-current-concepts-2329-8790-1000215. php?aid=59370. Published August 27, 2015. Accessed February 12, 2018
- Chiron D, Di Liberto M, Martin P, et al. Cell-cycle reprogramming for PI3K inhibition overrides a relapse-speci c C481S BTK mutation revealed by longitudinal functional genomics in mantle cell lymphoma. Cancer Discov. 2014;4(9):1022-1035. doi: 10.1158/2159-8290.CD-14-0098.
- Martin P, Blum K, Bartlett N, et al. A phase I trial of ibrutinib plus palbociclib in patients with previously treated mantle cell lymphoma. Blood. 2016;128. Abstract 150. bloodjournal.org/content/128/22/150?sso-checked=true.
- Zhao X, Bodo J, Sun D, et al. Combination of ibrutinib with ABT-199: synergistic effects on proliferation inhibition and apoptosis in mantle cell lymphoma cells through perturbation of BTK, AKT and BCL2 pathways. Br J Haematol. 2015;168:765-768. doi: 10.1111/bjh.13149.
- ABT-199 & Ibrutinib in Mantle Cell Lymphoma (AIM). clinicaltrials.gov/ct2/show/ NCT02471391?term=NCT02471391&rank=1. Updated August 31, 2015. Accessed February 16, 2018.
- Tam CS, Roberts AW, Andersen MA, et al. Combination ibrutinib (Ibr) and venetoclax (Ven) for the treatment of mantle cell lymphoma (MCL): primary endpoint assessment of the phase 2 AIM study. J Clin Oncol. 2017;35(suppl 15; abstr 7520). ascopubs.org/doi/abs/10.1200/JCO.2017.35.15_suppl.7520.
- A Study of Acalabrutinib in Combination With Rituximab Versus Ibrutinib Versus Acalabrutinib in Subjects With Relapsed or Refractory Mantle Cell Lymphoma. clinicaltrials.gov/ct2/show/NCT02735876?term=NCT02735876&rank=1. Updated May 13, 2016. Accessed February 16, 2018.
- A Study of Acalabrutinib (ACP-196) in Combination With Bendamustine and Rituximab in Subjects With Mantle Cell Lymphoma. clinicaltrials.gov/ct2/show/ NCT02717624?term=NCT02717624&rank=1. Updated January 30, 2018. Accessed February 16, 2018.
- Martin P, Ghione P, Dreyling M. Mantle cell lymphoma current standards of care and future directions. Cancer Treat Rev. 2017;58:51-60. doi: 10.1016/j.ctrv.2017.05.008.
- CART-19 Immunotherapy in Mantle Cell Lymphoma. clinicaltrials.gov/ct2/show/ NCT02081937?term=NCT02081937&rank=1. Updated March 11, 2014. Accessed February 16, 2018.
- A Phase 2 Multicenter Study Evaluating Subjects With Relapsed/Refractory Mantle Cell Lymphoma (ZUMA-2). clinicaltrials.gov/ct2/show/NCT02601313?term=NCT0 2601313&rank=1. Updated January 30, 2018. Accessed February 16, 2018.
- Hude I, Sasse S, Engert A, Brockelmann PJ. The emerging role of immune checkpoint inhibition in malignant lymphoma. Haematologica. 2017;102(1):30-42. doi: 10.3324/haematol.2016.150656.
- Pembrolizumab and Ibrutinib in Treating Patients With Relapsed or Refractory Non-Hodgkin Lymphoma. clinicaltrials.gov/ct2/show/NCT02950220?term=NCT0 2950220&rank=1. Updated January 29, 2018. Accessed February 16, 2018.
- Davids MS, Roberts AW, Seymour JF, et al. Phase I first-in-human study of venetoclax in patients with relapsed or refractory non-hodgkin lymphoma. J Clin Oncol. 2017;35(8):826-833. doi: 10.1200/JCO.2016.70.4320.
- The Study of Bendamustine, Rituximab, Ibrutinib, and Venetoclax in Relapsed, Refractory Mantle Cell Lymphoma. clinicaltrials.gov/ct2/show/NCT03295240?ter m=NCT03295240&rank=1. Updated September 27, 2017. Accessed February 16, 2018.
- Smith SM, Pitcher BN, Jung SH, et al. Safety and tolerability of idelalisib, lenalidomide, and rituximab in relapsed and refractory lymphoma: the Alliance for Clinical Trials in Oncology A051201 and A051202 phase 1 trials. Lancet Haematol. 2017;4(4):e176-e182. doi: 10.1016/S2352-3026(17)30028-5.
- Lenalidomide With or Without Idelalisib in Treating Patients With Relapsed or Refractory Mantle Cell Lymphoma. clinicaltrials.gov/ct2/show/NCT01838434?term =NCT01838434&rank=1. Updated January 30, 2018. Accessed February 16, 2018.
- Study of Safety and Ef cacy of BCL201 and Idelalisib in Patients With FL and MCL. clinicaltrials.gov/ct2/show/NCT02603445?term=NCT02603445&rank=1. Updated December 18, 2017. Accessed February 16, 2018.
- Le Gouill S, Thieblemont C, Oberic L, et al; LYSA Group. Rituximab after autologous stem-cell transplantation in mantle-cell lymphoma. N Engl J Med. 2017;377(13):1250-1260. doi: 10.1056/NEJMoa1701769.
- Rituximab With or Without Stem Cell Transplant in Treating Patients With Minimal Residual Disease-Negative Mantle Cell Lymphoma in First Complete Remission. C clinicaltrials.gov/ct2/show/NCT03267433?term=NCT03267433&ra nk=1. Updated December 8, 2017. Accessed February 16, 2018.
- Jacobson C. MCL old dog, new tricks: targeting CD20 in B cell non-hodgkin lymphomas. The Hematologist. January 9, 2018. hematology.org/Thehematologist/ Years-Best/8174.aspx. Accessed February 16, 2018.