Immune Checkpoint Inhibition in Esophagogastric Cancers
The poor prognosis for patients with esophagogastric cancers (EGC) requires the development of newer more effective therapies to further improve the treatment outcomes for this disease. Immunotherapy is a novel treatment strategy that is dramatically changing the treatment landscape for several types of cancers. Cytotoxic T lymphocyte antigen-4 (CTLA-4) antibody and antagonists of the programmed death (PD)-1/PD-ligand 1 are essential immune checkpoint inhibitors that suppress T-cell activation.
Geoffrey Ku, MD
Abstract
The poor prognosis for patients with esophagogastric cancers (EGC) requires the development of newer more effective therapies to further improve the treatment outcomes for this disease. Immunotherapy is a novel treatment strategy that is dramatically changing the treatment landscape for several types of cancers. Cytotoxic T lymphocyte antigen-4 (CTLA-4) antibody and antagonists of the programmed death (PD)-1/PD-ligand 1 are essential immune checkpoint inhibitors that suppress T-cell activation. Targeting of these immune checkpoints with monoclonal antibodies has shown clinical efficacy in several solid tumors which has led to their approval and use in routine clinical practice. In EGC early phase evaluation of immune checkpoint inhibitors has yielded encouraging results with multiple phase III studies currently ongoing. In this review, the biological rationale for the use of immune checkpoint inhibitors in cancer will briefly be described and the accumulating data concerning their use in EGC will be presented.
Introduction
In the United States, esophagogastric cancers (EGCs) are an uncommon but aggressive disease. In 2016, an estimated 26,370 patients were diagnosed with gastric cancer, with an estimated 10,370 deaths. Esophageal cancer cases will number 16,940, with an estimated 15,690 deaths during the same time period.1Approximately 50% of patients diagnosed with EGC present with overt metastatic disease and chemotherapy is the mainstay of palliation in this setting. The majority of patients will develop chemotherapy resistance, and treatment options beyond first or second line are limited in this disease. With the exception of trastuzumab with first-line chemotherapy for HER2-positive disease2and ramucirumab as monotherapy3or with paclitaxel chemotherapy4in the second-line setting, results of clinical trials utilizing targeted agents have not resulted in efficacious therapeutic options for patients.
Recent years have seen the treatment landscape for many cancers changed dramatically with the development of immune-directed therapies, specifically immune checkpoint inhibitors. There has been growing excitement among oncologists and patients alike for the use of checkpoint inhibition in EGC. The first checkpoint inhibitor drug to be approved by the FDA in 2011 was the anti-cytotoxic T lymphocyte antigen-4 (CTLA-4) antibody ipilimumab in advanced melanoma.5,6Since then, antagonists of the programmed death (PD)-1/PD-ligand 1 pathway have undergo extensive evaluation in multiple other solid tumors, with FDA approval of immune checkpoint inhibitors in melanoma, nonsmall cell lung cancer (NSCLC), urothelial carcinoma, renal cell carcinoma, squamous cell carcinoma of the head and neck, Merkel cell carcinoma, and Hodgkin lymphoma. In EGC, early-phase evaluation of immune checkpoint inhibitors has yielded encouraging results, culminating in ongoing phase III studies. Pembrolizumab which is an anti-PD-1 inhibitor was approved by the FDA in September 2017 for PD-L1–positive gastric cancer patients who have received 2 or more lines of therapy in the advanced setting. This accelerated approval was based on results of a phase II study and is contingent on results of a confirmatory trial. In this review, the biological rationale for the use of immune checkpoint inhibitors in cancer will briefly be described and the accumulating data concerning their use in EGC will be presented.
CTLA-4 and the PD-1/PD-L1/2 Pathway in Cancer
CTLA-4 is a protein that has high homology with CD28, which is known to be a co-stimulatory molecule expressed on T cells necessary to provide the secondary signal for T-cell activation.7Just like CD28, CTLA-4 binds to its cognate ligand, the B7 molecules (which are found on antigen presenting cells [APCs]), but with much higher affinity. However, unlike CD28, CTLA-4 expression is induced only when a T cell becomes activated. It then competes with CD28 for binding to the B7 molecules but leads to down-regulation and eventual abrogation of the immune response (Figure).
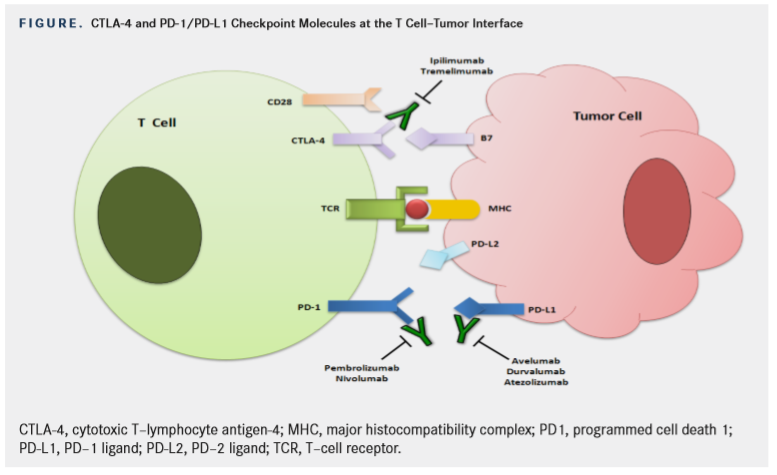
PD-1 is another negative immune checkpoint molecule.8PD-1 has 2 ligands, PD-L1 and -L2. PD-L2 is mostly expressed on APCs, while PD-L1 is expressed on numerous tissues, including immune and tumor cells. In the tumor microenvironment, PD-L1
expressed on tumor cells binds to PD-1 on activated T cells reaching the tumor. This delivers an inhibitory signal to those T cells, preventing them from killing target cancer cells.9Unlike CTLA-4, which is thought to be necessary for T-cell activation, the PD-1/PD-L1/2 pathway is thought to protect cells from T-cell attack.10
CTLA-4 Checkpoint Inhibitors in EGC
Immune checkpoint inhibition with antiCTLA-4 antibodies has been explored in the treatment of EGC. The 2 anti–CTLA-4 antibodies that have been evaluated are ipilimumab and tremelimumab.
Tremelimumab
The first immune checkpoint inhibitor to be studied in EGC is tremelimumab. In a phase II study, Ralph et al evaluated tremelimumab 15 mg/kg every 90 days in 18 patients with advanced esophageal, GEJ (or gastric adenocarcinoma). Fifteen patients had received prior first-line chemotherapy and 3 had received second-line treatment.11One patient achieved a partial response (PR, 6%) that was ongoing at 33 months of follow-up while 4 other patients achieved stable disease (SD, 22%). Although median time to progression (TTP) and overall survival (OS) were relatively disappointing (2.83 and 4.83 months respectively). One-third of patients were alive at12months. It should be noted that the dose of tremelimumab in this study is now considered subtherapeutic. In an ongoing study of tremelimumab (with or without the antiPD-L1 antibody durvalumab), the dose of tremelimumab monotherapy is 10 mg/kg every 4 weeks.
Ipilimumab
Data for ipilimumab has recently been published.12This was a randomized phase II study in which 114 patients with either a PR or SD to first-line fluoropyrimidine/platinum chemotherapy were randomized to best supportive care (BSC, which mostly consisted of continuation of the fluoropyrimidine) versus ipilimumab. The primary endpoint was immune-related progression-free survival (irPFS). The study was terminated early after the interim analysis due to the lack of demonstrable clinical activity with ipilimumab. The irPFS was only 2.9 months in patients who received ipilimumab versus 4.9 months for patients who continued on fluoropyrimidine maintenance chemotherapy. The median OS was similar in both groups (12.7 vs 12.1 months). Toxicities were also higher in the ipilimumab versus BSC arm (72% vs 56%) and included pruritus (32%), diarrhea (25%), fatigue (23%) and rash (18%).
PD-1 and PD-L1 Checkpoint Inhibitors in EGC
There are a number of antibodies targeting the PD-1 and PD-L1 pathway now approved for the treatment of various cancers. Many of these anti-PD-1 and antiPD-L1 antibodies have been evaluated in EGC, including pembrolizumab, nivolumab, avelumab and durvalumab. The following section along withTables 1 and 2provides a summary of the clinical trial data involving these PD-1/PD-L1 inhibitors in EGC.
Pembrolizumab (PD-1 Inhibitor)
The KEYNOTE-012 study was a multicenter, nonrandomized, open-label, multicohort phase Ib trial evaluating single-agent pembrolizumab (10 mg/kg every 2 weeks or a 200-mg fixed dose every 3 weeks) that evaluated patients with PD-L1positive recurrent or metastatic gastric/GEJ adenocarcinoma.13The cut-off for positivity was ≥1% membrane staining of tumor or peri-tumoral mononuclear inflammatory cells. Of 162 screened tumors, 65 (40%) were noted to be PD-L1 positive. Thirty-nine patients enrolled in the study. Nineteen patients were from Asia and the remainder was from the rest of the world. Patients were heavily pretreated and 2/3 had received ≥2 prior therapies. The confirmed overall response rate (ORR) was 22% for all patients; 4 of these 8 patients had ongoing responses at the time of data analysis and the median duration of response was 40 weeks. Median progression-free survival (PFS) was 1.9 months and median OS was 11.4 months; the 6- and 12-month OS rates were 66% and 42%, respectively.
In the similarly designed KEYNOTE 028 study, 23 patients with PD-L1positive esophageal cancer were treated, 17 with squamous cell cancer (SCC), 5 with adenocarcinoma and 1 with a mucoepidermoid histology.14,15Among the 90 screened patients, the PD-L1 positivity rate was 41%, virtually identical to the rate in GEJ/gastric adenocarcinoma. This was again a heavily pretreated group, with 87% of patients receiving ≥2 prior therapies. Seven of 23 patients (30%) had a PR, with 5 of the PRs ongoing at the time of data analysis. The median duration of response was 40.0 weeks. Six- and 12-month PFS were 30.4% and 21.7%, respectively.
The KEYNOTE-059 study is an open-label, multicohort phase II study of patients with advanced gastric/GEJ adenocarcinoma.16Patients in cohort 1 had received ≥2 prior lines of therapy and HER2/ positive disease was permitted if the patient had received prior trastuzumab-based therapy. Single- agent pembrolizumab was administered at 200 mg every 3 weeks. Patients in cohort 2 had received no prior therapy for advanced disease and were administered pembrolizumab 200 mg plus 800 mg/m2of 5-fluorouracil (5-FU; or 1000 mg/m2of capecitabine in Japan) plus 80 mg/m2of cisplatin every 3 weeks for 6 cycles, followed by pembrolizumab plus 5-FU/ capecitabine maintenance for up to 2 years or until progression. Lastly, patients in cohort 3 were also treatment naïve, similar to cohort 2, but must have had PD-L1-positive disease.
Results of cohort 1, which enrolled 259 patients were recently presented.16Overall, 51.7% of patients had received 2 prior lines of therapy, and 29% and 19.3% had received 3 or ≥4 prior lines of therapy, respectively. After a median follow-up of 5.8 months, the overall ORR among the 259 patients was 11.2%, with a complete response (CR) rate of 2.3% and a PR rate of 9.3%. The median duration of response (DOR) was 8.4 months. Patients with PD-L1positive tumors (n = 148) had an ORR of 15.5%. The ORR was 6.4% in the PD-L1–negative group (n = 109). The median DOR in the PD-L1–positive group was 16.3 versus 6.9 months in those with PDL1 negative disease. Patients treated in the third-line setting had an ORR of 16.4% compared with 6.4% for patients with ≥4 lines of therapy. The PFS for all 259 patients was 2 months with a median OS of 5.6 months and a 12-month OS rate of 23.4%.
These results are encouraging and suggest that pembrolizumab has promising antitumor activity in pretreated advanced gastric/GEJ cancer. Based on the findings of the KEYNOTE-059 study, the FDA has recently approved pembrolizumab for the treatment of patients with PD-L1positive recurrent or advanced gastric or GEJ adenocarcinoma who have received 2 or more lines of chemotherapy, including fluoropyrimidine and platinum-containing chemotherapy, and, if appropriate, HER2 therapy. The accelerated approval of pembrolizumab for this indication is contingent on the results of a confirmatory trial.
Preliminary efficacy and safety data from 25 enrolled patients in cohort 2 of the KEYNOTE-059 study have also been presented in abstract form.17After a median follow-up of 12.2 months, grade 3-4 treatment-related adverse events (TRAEs) occurred in 76% of patients, with no fatal events. ORR was 60% in all patients and was 68.8% in PD-L1 positive patients and 37.5% in PD-L1 negative patients. Median DOR was 4.6 months in all patients, while median PFS and OS were 6.6 months and
13.8 months, respectively. These early data suggest that combination of pembrolizumab and cisplatin/5FU chemotherapy has a manageable toxicity profile and encouraging antitumor activity as first-line therapy for patients with advanced gastric cancer. It is interesting to note that the ORR in patients with PD-L1 negative tumors was the same as would be expected with a fluoropyrimidine/platinum doublet alone and the benefit of adding an antiPD-1 antibody to first-line chemotherapy will have to await several ongoing phase III studies (Table 2).
There are reasons to believe that adding immunotherapy to chemotherapy can be a beneficial strategy. A recently published randomized phase II study of first-line pembrolizumab plus carboplatin/pemetrexed in NSCLC showed an improved response with similar toxicity to chemotherapy alone.18However, survival data are still pending. Based on this study, pembrolizumab has received FDA approval in combination with carboplatin/pemetrexed in non-squamous NSCLC. Given these positive results in NSCLC, it is hoped that they might be replicated in advanced EGC.
Nivolumab (PD-1 Inhibitor)
CheckMate-032 was a phase I/II open-label study of the safety and activity of nivolumab alone or with ipilimumab in advanced and metastatic solid tumors.19This study enrolled 160 patients with advanced/metastatic gastric or gastroesophageal cancer who had progressed while receiving standard chemotherapy, most of whom (79%) received ≥2 regimens. Patients were randomized to receive 3 mg/kg of nivolumab every 2 weeks (N3), 1 mg/kg of nivolumab plus 3 mg/kg of ipilimumab (N1 plus I3) or 3 mg/kg of nivolumab plus 1 mg/kg of ipilimumab (N3 plus I1) every 3 weeks for 4 cycles, followed by 3 mg/kg of nivolumab every 2 weeks until disease progression or intolerable toxicity. Updated results concerning the 59 patients enrolled to the N3 cohort were presented in abstract form and suggest similar activity to pembrolizumab.20The ORR was 12%, with a median time-to-response of 1.6 months and DOR of 7.1 months in the responders. Median OS was 6.2 months for the entire group and the 12-month survival rate was 39%. PD-L1 positivity was assessed using a cut-off of ≥1% for immunohistochemistry (IHC) positivity. The ORRs in patients with PD-L1positive and negative tumors were 19% and 12%, respectively.
Very similar activity was also noted for nivolumab in a Japanese study of 64 patients with esophageal SCC, who had received a median of 3 prior therapies.21The ORR was 17.2%, including a CR in 1 patient. Median PFS was 1.5 months and median OS was 10.8 months.
The largest study to date evaluating nivolumab in EGC is the ATTRACTION-2 trial.22This was a multicenter, double-blind, randomized phase III East Asian study of 493 patients who had received >2 prior regimens. Patients were randomized 2:1 to nivolumab versus placebo. The median OS was 5.32 months with nivolumab versus 4.14 months with placebo [HR 0.63, P <.0001]. The 6-month OS rate was 46.4% versus 34.7% and 12-month OS rate was 26.6% versus 10.9% in favor of nivolumab. The ORR was 11.2% versus 0%, with a median DOR to nivolumab being 9.53 months. The PFS was 1.65 versus 1.45 months [HR 0.60, P <.0001). Nivolumab has recently received approval in Japan as salvage therapy for chemotherapy refractory EGC.
If we compare outcomes from the nivolumab ATTRACTION-2 study with cohort 1 of the pembrolizumab KEYNOTE-059, we observe nearly identical results for OS 5.32 months (ATTRACTION-2) versus 5.6 months (KEYNOTE-059). Similarly, ORR was 11.2% versus 11.9%, 12-month OS 26.6% versus 23.4% and PFS 1.65 months versus 2 months, respectively. Taken together, these findings confirm that PD-1 inhibition is an effective treatment approach in this disease setting.
Avelumab (PD-L1 Inhibitor)
Avelumab has been evaluated in a phase Ib JAVELIN study23that enrolled patients with gastric cancer and GEJ who had progressed on ≥ 2 lines of prior therapy (n = 20). Patients who had received 1 line of chemotherapy but had not yet progressed (switch-maintenance) were also enrolled (n = 55). For patients in the ≥2 lines of prior therapy cohort the ORR was 15% (3 of 20 patients). PD-L1 expression (≥1% cutoff) was evaluable in 12 of 20 patients in this cohort. Median PFS was 36.0 weeks (95% CI: 6.0, 36.0) for PD-L1positive and 11.6 weeks (2.1, 21.9) for PD-L1 negative tumors. Avelumab is currently being evaluated in the third-line setting versus BSC (NCT02625263) and as maintenance therapy after primary chemotherapy versus continuation of chemotherapy in the first-line setting (NCT02625610).
Durvalumab (PD-L1 Inhibitor)
Finally, an abstract presentation has also shown responses for the PD-L1 antibody durvalumab in 16 patients with EGC, where 4 patients had a PR.24
Nivolumab and Ipilimumab (Combination PD-1/ CTLA-4 Inhibition)
The only data for combination immune checkpoint blockade in EGC comes from the Checkmate 032 study, which was recently presented in abstract form.20In addition to the 59 patients treated with nivolumab alone (and discussed above), there were an additional 2 cohorts that received different doses of nivolumab (N) together with ipilimumab (I). Fortynine patients were enrolled to the N1+I3 cohort and 52 patients enrolled to the N3+I1 cohort. Baseline characteristics in these other 2 groups were similar to the nivolumab-only arm. The highest ORR (24%) was reported for patients in the N1+I3 group. The ORR for the N3+I1 group was 8%. The N1+I3 group contained 10 PD-L1positive tumors with an ORR of 40% versus 22% for PD-L1 negative tumors. Survival data in these relatively small groups of patients appeared comparable; 6.2 versus 6.9 versus 4.8 months for N3, N1+I3, and N3+I1 cohorts, respectively. Grade ≥3 toxicities were highest in the N1+I3 group (35% vs 5% in the N3 group). The most common grade 3/4 toxicities seen in this group were diarrhea 10%, increased alanine aminotransferase (14%) and increased aspartate aminotransferase (10%). Nevertheless, this dose has been selected as the basis for a proposed phase III study.
These results have to be interpreted with caution not only because of the small patient numbers but also because patients were not randomized to the treatment arms but were instead enrolled sequentially. Unless validated, these findings also raise puzzling questions: Why does the addition of ipilimumab 1 mg/kg to nivolumab 3 mg/kg result in a lower ORR versus nivolumab monotherapy? Similarly, why does the combination of N1+I3 result in a higher ORR versus N3 without any hint of a survival benefit? Overall, this study justifies the ongoing phase III Checkmate 649 study (Table 3) but not the routine use of combination antiPD-1 and anti–CTLA-4 therapy.
Pembrolizumab and Ramucirumab (PD-1 and VEGF Inhibition)
Ramucirumab is a monoclonal antibody against VEGFR2, which is approved as a single agent and in combination with paclitaxel for second-line therapy in EGC. A multicohort phase Ia/b study was presented in abstract form by Chau et al and is the first to evaluate the simultaneous targeting of both PD-1 and VEGFR2 in EGC.25Forty-one patients with advanced gastric or GEJ adenocarcinomas were enrolled to 3 cohorts: previously treated with chemotherapy (cohorts A and B) or chemotherapy-näive (cohort A2). Ramucirumab was administered at 8 mg/kg on days 1 and 8 (cohorts A and A2) or 10 mg/ kg on day 1 (cohort B) with pembrolizumab 200 mg every 3 weeks. Response rate in cohort A and B was 7%. PFS and OS rates at 6 months were 22.4% and 51.2%, respectively. Eighteen patients were enrolled to A2 cohort with an ORR of 17%. Any grade toxicity was 80%, with a grade 3/4 toxicity rate of 24%, most commonly colitis (7%) and hypertension (7%). Again, these small numbers preclude any specific conclusion but the activity noted so far does not seem to indicate any particular advantage to adding ramucirumab to pembrolizumab.
Immune Related Toxicity from Checkpoint Inhibitors
Checkpoint inhibitors can induce immune-related AEs that resemble autoimmune-like conditions. When we use immune checkpoint inhibitors to target cancer, the goal is to achieve a hyperactivated T-cell response directed toward the tumor cell. However, this hyperactivated T-cell response can also result in reactivity directed against normal tissue. Immunerelated serious AEs can affect multiple organs of the body including skin, the gastrointestinal tract, the kidneys, both peripheral and central nervous systems, liver, lymph nodes, eyes, pancreas, and the endocrine system.26,27The immune-related adverse events observed across the trials of checkpoint inhibitors in EGC have been similar to published data in other disease types with no new safety signals observed.28 PD-1 and PD-L1 inhibitors appear to be better tolerated compared with CTLA-4 inhibition, a finding that has been observed across studies of these agents in other cancers.28The highest rate of AEs was seen in the Checkmate 032 study with the combination of nivolumab 1 mg/kg and ipilimumab 3 mg/kg.20Table 1 summarizes the grade 3-4 AEs from checkpoint inhibition studies in EGC to date.
Biomarkers to Predict Response to Immune Checkpoint Inhibition
The results of the studies above uniformly suggest that less than 25% of patients who receive immune checkpoint inhibitors derive significant benefit. Most studies report a median PFS of less than 2 months, even in the setting of encouraging OS, suggesting that most patients are rapidly progressing on these treatments. Therefore, the identification of biomarkers to select patients most likely to benefit from these expensive and potentially toxic agents is a priority.
PD-L1 expression occurs in approx 40% to 60% of gastric/GEJ cancers.13,16PD-L1 status by IHC had been hypothesized to be a biomarker to select patients for immune-directed therapy with PD-1/PDL1 inhibition. Unfortunately this is not the case and PD-L1 has been proved to be an imperfect biomarker in EGC and many other cancers. Although PD-L1 positive tumors appear more likely to respond to treatment with anti–PD-1/PD-L1 antibodies, many of the studies above suggest the possibility of response and disease control even for patients with PD-L1negative tumors. The situation is further complicated by the fact that there are currently several antibodies that are available for PD-L1 testing that have not been directly compared against each other. Therefore, many ongoing and phase III studies are enrolling patients irrespective of the tumor PD-L1 status.
Another approach is to identify a genetic signature within the tumor and/or peritumoral tissue that may correlate with an increased chance of benefit from immune checkpoint inhibitors. In the KEYNOTE-012 study with pembrolizumab, a 6-gene signature of interferon-gamma genes (CXCL9, CXCL10, IDO1, IFNG, HLA-DRA, andSTAT1) was assessed using gene expression profiling of RNA isolated from tumor samples.29,30 There was a trend between a higher interferon-gamma signature score and response but it did not achieve statistical significance (P= .070), possibly a reflection of the small numbers involved (only 30 tumor samples could be tested). One benefit of this gene signature is that it may be more reproducible and robust than PDL1 testing and efforts continue to evaluate it in EGC and other cancers.
Finally, there are also ongoing efforts to correlate response and benefit on these studies with the 4 subtypes of gastric cancer identified by the Cancer Genome Atlas (TCGA): Epstein-Barr virus (EBV) positive, microsatellite unstable (MSI), genomically stable (GS), and chromosomal instability (CIN).29Of these subtypes, both the EBV and MSI groups may be more responsive to immune checkpoint inhibition.
The MSI subgroup accounts for 22% of patients with gastric cancer. It is characterized by MLH1 promoter hypermethylation, which is associated with an elevated mutation rate. A cancer may be MSI through either a somatic or germline mutation. Although the TCGA analysis reported such a relatively high incidence of the MSI subgroup, it is critical to note that this analysis was restricted to patients with operable tumors. In the metastatic setting, the incidence of mismatch repair protein deficient (dMMR) or MSI tumors is much lower. Our anecdotal experience in over 200 patients suggests a dMMR/ MSI incidence of less than 5%; we have tested tumor samples from greater than 200 patients and only 4 patients were found to be dMMR/MSI.31
Pembrolizumab has shown activity in MSI-high colorectal cancer.32Recent data also suggest significant activity in other dMMR gastrointestinal cancers, including gastric cancer.33In May 2017, the FDA granted accelerated approval to pembrolizumab for adult and pediatric patients with unresectable or metastatic, MSI-H or dMMR solid tumors that have progressed following prior treatment and who have no satisfactory alternative treatment options. The approval was based on data from 149 patients with MSI-H or dMMR cancers enrolled across 5 singlearm clinical trials.34Ninety patients had colorectal cancer and 59 patients were diagnosed with one of 14 other cancer types, of which 9 patients had EGC. The ORR was 39.6% with a DOR of ≥6 months being 78% in the total population. There were 11 CRs and 48 PRs. Of the 9 EGC patients, The ORR was 56% with 5 out of the 9 patients achieving a PR.
To ensure MSI-H patients gain access to checkpoint inhibitor therapy, it is prudent to test all patients with EGC for dMMR or MSI status irrespective of a family pedigree that might suggest a germline mutation. It is likely that, as next-generation sequencing becomes part of routine clinical practice, mutational burden will identify patients with MSI-H tumors who are most likely to benefit from checkpoint inhibitors.
Future Directions
Based on the results above, numerous phase III studies are ongoing or planned, as noted in Table 3. Of note, the KEYNOTE-059 study, which has completed accrual, included a first-line arm in which patients received pembrolizumab in combination with 5-fluorouracil/cisplatin. This combination is being further tested in the phase III KEYNOTE-062 study.35This is a study of pembrolizumab as first-line treatment for patients with advanced PD-L1positive gastric or GEJ adenocarcinoma. Participants are randomly assigned to one of the 3 treatment arms: pembrolizumab as monotherapy, or 5-FU/cisplatin with or without pembrolizumab. This study has also completed accrual and its results will therefore determine if there is a benefit for combination immune checkpoint blockade and chemotherapy in the first-line therapy of EGC.
In addition to the above first-line studies with pembrolizumab, Checkmate 649 is a phase III study which is currently enrolling advanced gastric or GEJ tumor patients and randomizing them to ipilimumab/nivolumab versus nivolumab/FOLFOX versus FOLFOX.
Also of interest is the Checkmate 577 study, which is evaluating the benefit of adjuvant nivolumab versus placebo in patients with locally advanced esophageal/GEJ tumors (both adenocarcinomas and SCC), who have undergo chemoradiation and surgery but are found to have persistent disease (pathologically defined as ypTanyN+ or ypT1-4Nanyany).36
Finally, a phase Ib/II study is evaluating combination immune checkpoint blockade, this time with a PD-L1 inhibitor (durvalumab) and an antiCTLA-4 antibody (tremelimumab) in the second- and thirdline setting.37
These studies represent only a fraction of ongoing or planned phase I/II studies that will combine immune checkpoint inhibitors with other immunotherapy drugs, chemotherapy, targeted therapies, or locoregional approaches (such as radiation or ablative procedure). Many of these studies are specifically enrolling patients with EGC but also include studies that are enrolling these patients in dose- expansion cohorts.
Conclusions
The evaluation of immune checkpoint inhibitors in solid tumors in general but also in EGC has occurred at a breathtaking pace. Two studies, ATTRACTION-2 (nivolumab) and KEYNOTE-059 (pembrolizumab) have now confirmed the activity of an antiPD-1 antibody in the chemorefractory setting, resulting in regulatory approval (pembrolizumab in the United States and nivolumab in Japan) in this indication. Results of phase III studies are awaited eagerly and it is hoped that they will establish a new treatment paradigm in EGC. These drugs are not without both economic and clinical toxicity, with responses rates in a small albeit significant population of patients. Therefore, it is imperative that we attempt to identify patients most likely to benefit from these therapies through ongoing correlative efforts and the next generation of studies evaluating combinatorial strategies.
References
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017.CA Cancer J Clin. 2017;67(1):730. doi: 10.3322/caac.21395.
- Bang YJ, Van Cutsem E, Feyereislova A, et al; ToGA Trial Investigators. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial.Lancet. 2010;376(9742):687697. doi: 10.1016/S0140-6736(10)61121.
- Fuchs CS, Tomasek J, Yong CJ, et al; REGARD Trial Investigators. Ramucirumab monotherapy for previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (REGARD): an international, randomised, multicentre, placebo-controlled, phase 3 trial.Lancet. 2014;383(9911):31-39.doi: 10.1016/S0140-6736(13)61719-5.
- Wilke H, Muro K, Van Cutsem E, et al; RAINBOW Study Group. Ramucirumab plus paclitaxel versus placebo plus paclitaxel in patients with previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (RAINBOW): a double-blind, randomised phase 3 trial.Lancet Oncol. 2014;15(11):1224-1235. doi: 10.1016/S1470-2045(14)70420-6.
- Hodi FS, O’Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma.N Engl J Med. 2010;363(8):711-723. doi: 10.1056/NEJMoa1003466.
- Robert C, Thomas L, Bondarenko I, et al. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma.N Engl J Med. 2011;364(26):2517-2526. doi: 10.1056/NEJMoa1104621.
- Krummel MF, Allison JP. CD28 and CTLA-4 have opposing effects on the response of T cells to stimulation.J Exp Med. 1995;182(2):459-465.
- Freeman GJ, Long AJ, Iwai Y, et al. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation.J Exp Med. 2000;192(7):1027-1034.
- Zou W, Chen L. Inhibitory B7-family molecules in the tumour microenvironment.Nat Rev Immunol. 2008;8(6):467-477. doi: 10.1038/nri2326.
- Sharma P, Allison JP. The future of immune checkpoint therapy.Science. 2015;348(6230):56-61. doi: 10.1126/science.aaa8172.
- Ralph C, Elkord E, Burt DJ, et al. Modulation of lymphocyte regulation for cancer therapy: a phase II trial of tremelimumab in advanced gastric and esophageal adenocarcinoma.Clin Cancer Res. 2010;16(5):1662-1672. doi: 10.1158/1078-0432.CCR-09-2870.
- Bang YJ, Cho JY, Kim YH, et al. Efficacy of sequential ipilimumab monotherapy versus best supportive care for unresectable locally advanced/metastatic gastric or gastroesophageal junction cancer.Clin Cancer Res. 2017;23(19):5671-5678. doi: 10.1158/1078-0432.CCR-17-0025.
- Muro K, Chung HC, Shankaran V, et al. Pembrolizumab for patients with PD-L1-positive advanced gastric cancer (KEYNOTE-012): a multicentre, open-label, phase 1b trial.Lancet Oncol. 2016;17(6):717-726. doi: 10.1016/S1470-2045(16)00175-3.
- Doi T, Piha-Paul SA, Jalal SI, et al. Pembrolizumab (MK-3475) for patients (pts) with advanced esophageal carcinoma: preliminary results from KEYNOTE-028.J Clin Oncol. 2015;33(suppl 15):4010.
- Doi T, Piha-Paul SA, Jalal SI, et al, Updated results for the advanced esophageal carcinoma cohort of the phase Ib KEYNOTE-028 study of pembrolizumab (MK3475).J Clin Oncol. 2016;34(suppl 4):7.
- Fuchs CS, Doi T, Jang RW-J, et al. KEYNOTE-059 cohort 1: efficacy and safety of pembrolizumab (pembro) monotherapy in patients with previously treated advanced gastric cancer.J Clin Oncol. 2017;35(suppl 15):4003.
- Bang Y-J, Koshiji M, Fuchs CS, et al. KEYNOTE-059 cohort 2: safety and efficacy of pembrolizumab (pembro) plus 5-fluorouracil (5-FU) and cisplatin for first-line (1L) treatment of advanced gastric cancer.J Clin Oncol.2017;35(suppl 15):4012.
- Langer CJ, Gadgeel SM, Borghaei H, et al; KEYNOTE-021 Investigators. Carboplatin and pemetrexed with or without pembrolizumab for advanced, non-squamous non-small-cell lung cancer: a randomised, phase 2 cohort of the open-label KEYNOTE-021 study.Lancet Oncol. 2016;17(11):1497-1508. doi: 10.1016/S1470-2045(16)30498-3.
- Janjigian YY, Bendell JC, Calvo E, Kim JW, Ascierto PA, Sharma P. CheckMate-032: phase I/II, open-label study of safety and activity of nivolumab (nivo) alone or with ipilimumab (ipi) in advanced and metastatic (A/M) gastric cancer (GC).J Clin Oncol. 2016;34(suppl 15):4010.
- Janjigian YY, Ott PA, Calvo E, Kim JW, Ascierto PA, Sharma P. Nivolumab ± ipilimumab in pts with advanced (adv)/metastatic chemotherapy-refractory (CTx-R) gastric (G), esophageal (E), or gastroesophageal junction (GEJ) cancer: CheckMate 032 study.J Clin Oncol. 2017;35(suppl 15):4014.
- Kojima T, Hara H, Yamaguchi K, et al. Phase II study of nivolumab (ONO-4538/ BMS-936558) in patients with esophageal cancer: preliminary report of overall survival.J Clin Oncol. 2016;34(suppl; abstr TPS175).
- Kang Y-K, Satoh T, Ryu M-H, Chao Y, Kato K, Chung HC. Nivolumab (ONO-4538/ BMS-936558) as salvage treatment after second or later-line chemotherapy for advanced gastric or gastro-esophageal junction cancer (AGC): a double-blinded, randomized, phase III trial.J Clin Oncol.2017;35(suppl 4S; abstr 2).
- Chung HC, Arkenau H-T, Wyrwicz L, et al. Safety, PD-L1 expression, and clinical activity of avelumab (MSB0010718C), an antiPD-L1 antibody, in patients with advanced gastric or gastroesophageal junction cancer.J Clin Oncol. 2016;34(suppl 4; abstr 167).
- Segal NH, Hamid O, Hwu W, et al. 1058PD: a phase I multi-arm dose-expansion study of the antiprogrammed cell death-ligand-1 (PD-L1) antibody MEDI4736: preliminary data.Ann Oncol. 2014;25(suppl 4):iv365. doi: 10.1093/annonc/mdu342.11.
- Chau I, Bendell JC, Calvo E, Santana-Davila R, Arkenau H-T, Mi G. Ramucirumab (R) plus pembrolizumab (P) in treatment naive and previously treated advanced gastric or gastroesophageal junction (G/GEJ) adenocarcinoma: a multi-disease phase I study.J Clin Oncol. 2017;35(suppl 15; abstr 4046).
- Michot JM, Bigenwald C, Champiat S, et al. Immune-related adverse events with immune checkpoint blockade: a comprehensive review.Eur J Cancer. 2016;54:139-148. doi: 10.1016/j.ejca.2015.11.01.
- Naidoo J, Page DB, Li BT, et al. Toxicities of the antiPD-1 and anti–PD-L1 immune checkpoint antibodies.Ann Oncol. 2015;26(12):2375-2391. doi: 10.1093/annonc/mdv383..
- Osta BEE, Hu F, Sadek RF, Chintalapally R, Tang S-C. Immune checkpoint inhibitors (ICI): a meta-analysis of immune-related adverse events (irAE) from cancer clinical trials.J Clin Oncol. 2016;34(suppl 15):e14562.
- Cancer Genome Atlas Research Network. Comprehensive molecular characterization of gastric adenocarcinoma. Nature. 2014;513(7517):202-209. doi: 10.1038/nature13480.
- Muro K, Chung HC, Shankaran V, et al. Pembrolizumab for patients with PD-L1positive advanced gastric cancer (KEYNOTE-012): a multicentre, open-label, phase 1b trial.Lancet Oncol.2016;17(6):717-726. doi: 10.1016/S1470-2045(16)00175-3.
- Ku GY, Sanchez-Vega F, Chatila W, Margolis M, Fein C, Ilson DH. Correlation of benefit from immune checkpoint inhibitors with next gen sequencing (NGS) profiles in esophagogastric cancer (EGC) patients.J Clin Oncol. 2017;35(suppl 15):4025.
- Le DT, Uram JN, Wang H, et al. PD-1 blockade in tumors with mismatch-repair deficiency.N Engl J Med. 2015;372(26):2509-2520. doi: 10.1056/NEJMoa1500596.
- Le DT, Uram JN, Wang H, et al. PD-1 blockade in mismatch repair deficient noncolorectal gastrointestinal cancers.J Clin Oncol.2016;34(suppl 4, abstr 195).
- Keytruda [prescribing information]. Kenilworth, NJ: Merck & Co; 2017.
- Tabernero J, Bang Y-J, Fuchs CS, et al. KEYNOTE-062: phase III study of pembrolizumab (MK-3475) alone or in combination with chemotherapy versus chemotherapy alone as first-line therapy for advanced gastric or gastroesophageal junction (GEJ) adenocarcinoma.J Clin Oncol.2016;34(suppl 4S, abstr TPS185).
- Kelly RJ, Lockhart AC, Jonker DJ, Melichar B, Andre T, Chau I. CheckMate 577: a randomized, double-blind, phase 3 study of nivolumab (Nivo) or placebo in patients (Pts) with resected lower esophageal (E) or gastroesophageal junction (GEJ) cancer.J Clin Oncol.2017;35(suppl 4, abstr TPS212).
- Kelly RJ, Chung K, Gu Y, et al. Phase Ib/II study to evaluate the safety and antitumor activity of durvalumab (MEDI4736) and tremelimumab as monotherapy or in combination, in patients with recurrent or metastatic gastric/gastroesophageal junction adenocarcinoma.J Immunother Cancer.2015;3(suppl 2):P157.

Novel Approaches Focus on Limiting Toxicity in Older Patients With ALL
April 22nd 2024The major challenges for clinicians treating older patients with acute lymphoblastic leukemia surround the emergence of resistance to existing therapies and the toxicities associated with current chemotherapies.
Read More
Ornstein Advises on Starting Dose and Management of Lenvatinib in RCC
April 21st 2024During a Case-Based Roundtable® event, Moshe Ornstein, MD, MA, provided guidance on dosing and toxicity concerns in a patient treated with lenvatinib plus pembrolizumab for advanced renal cell carcinoma.
Read More
Rugo Surveys Peers on Treatment After Metastatic Relapse of HR+, HER2– Breast Cancer
April 20th 2024During a Case-Based Roundtable® event, Hope S. Rugo, MD, FASCO, moderated a discussion on treatment options for a patient whose breast cancer recurred several years after adjuvant therapy.
Read More