Safety, Efficacy Results Bolster the Use of LuPSMA in Advanced Prostate Cancer
The novel targeted radiation therapy, lutetium 177 prostate-specific membrane antigen, demonstrated safety and therapeutic efficacy in 22 patients with metastatic castration-resistant prostate cancer, based on findings presented during the ESMO 2019 International Congress on Targeted Anticancer Therapies.
1
“In properly selected patients with mCRPC, LuPSMA may become an effective therapeutic option,” Manoj Gupta, DRM, told audience mem- bers during the conference held in Paris, France. Patients received 1 cycle of LuPSMA in a 50-mL normal saline infusion over 15 minutes. To be included in the study, patients had to have histologically proven adenocarcinoma and previous treatment with at least first-line antiandrogen therapy and docetaxel.
Despite advances in systemic therapies, men presenting with metastatic prostate cancer have poor outcomes, with overall survival (OS) of less than 5 years, Gupta said.2Further, men who progress to mCRPC demonstrate an OS of less than 3 years.3PSMA is overexpressed in cancer cells, with higher expression in higher-grade and metastatic castration-resistant prostate cancer.4LuPSMA is a radiolabeled small molecule that binds with high affinity to PSMA, enabling tumor-targeted delivery of beta radiation.
The investigators determined ECOG performance status (0-5), visual analogue scale for pain (VAS; 0-10), and analgesic quantification scale (AQS; 0-6). “The mean ECOG score before treatment was 3.3. After the 8-week postPSMA-targeted radioligand therapy (PRLT) period, ECOG [score] was 3.1. All 3 efficacy parameters were statistically significant,” Gupta said, a consultant for the department for nuclear medicine, Rajiv Gandhi Cancer Institute and Research Centre, Delhi, India (TABLE).
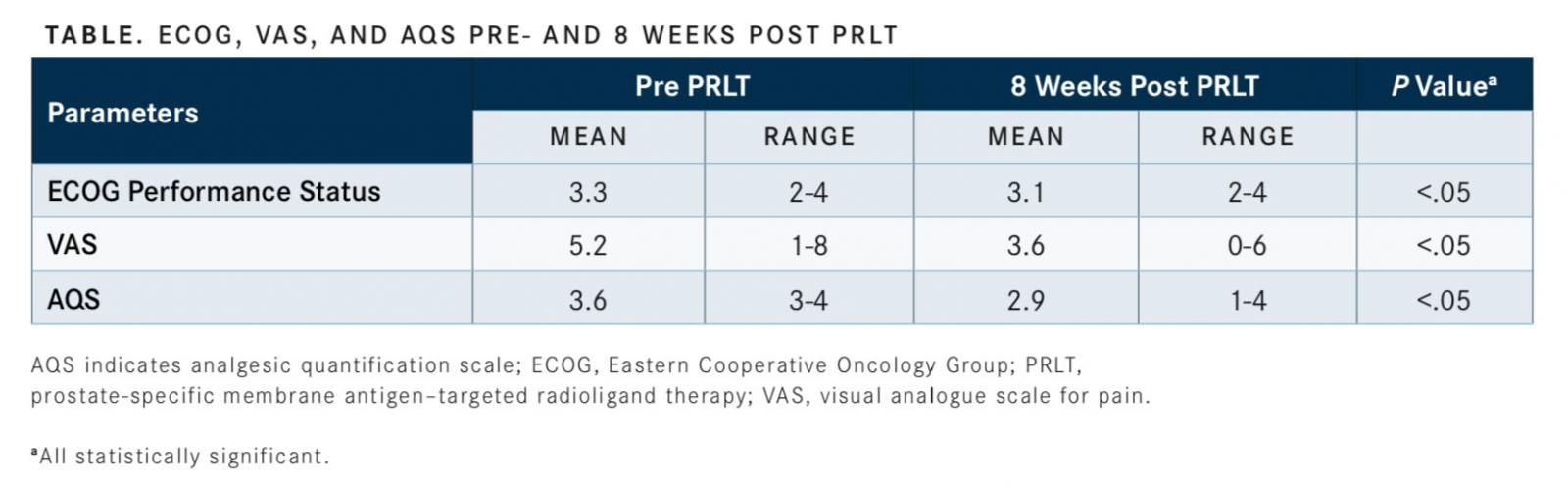
Gupta added that 27.3% of patients (6/22) had an improvement in their ECOG score of 1 point, just over 54% (12/22) showed a 2-point or more improvement in VAS, and 77.3% (17/22) had a 1-point improvement in VAS.
Partial response (PR), progressive disease (PD), and stable disease (SD) were determined based on prostate-specific antigen (PSA) levels that were obtained pre and 8 weeks post PRLT. PR was defined as a ≥50% drop in PSA, PD was defined as a ≥25% increase in PSA, and SD was defined as changes between PR and PD.
Initially, 22 patients received a median dose of 6.88 gigabecquerel (GBq) units of radioactivity. Five patients (22.7%) demonstrated a PR, 13 (59.1%) had SD, and 4 (18.2%) had PD. Eight patients (36.4%) demonstrated a greater than 30% drop in PSA. When the full dose of 7.4 GBq of LuPSMA was administered to 18 patients, the investigators noted 5 patients (27.8%) with a PR, 12 (66.7%) had SD, and 1 (5.6%) had PD.
Eight patients (44.4%) had a greater than 30% drop in PSA levels. “The higher dose had a better response compared with the median dose of 6.8 GBq,” Gupta said.
Toxicity parameters were determined using hemoglobin, platelets, creatinine, bilirubin, and glomerular filtration rate levels. “We found that only the hemoglobin values, pre and post, were statistically significant. The other measurements showed no significant differences.”
Gupta noted that when further analysis of hemoglobin toxicity was undertaken, half of the patients before treatment had grade 2 toxicities, but “no patient with normal or grade 1 hemoglobin toxicity before treatment with LuPSMA developed grade 3 hemoglobin toxicity.” Five of 22 patients (22.7%) who initially had grade 2 toxicity progressed to grade 3 after treatment.

Creating Solutions for a 'Continual State of Transition' in Cancer Care
April 15th 2024In a Peers & Perspectives in Oncology feature article, we focus on the importance of the transition-of-care process for patients with cancer as they move from the inpatient to outpatient setting, as well as between lines of therapy with comments from Marc J. Braunstein, MD, PhD, and Michael Shusterman, MD.
Read More