Barriers to Interpreting Results in Molecular Profiling Exist Among Oncologists
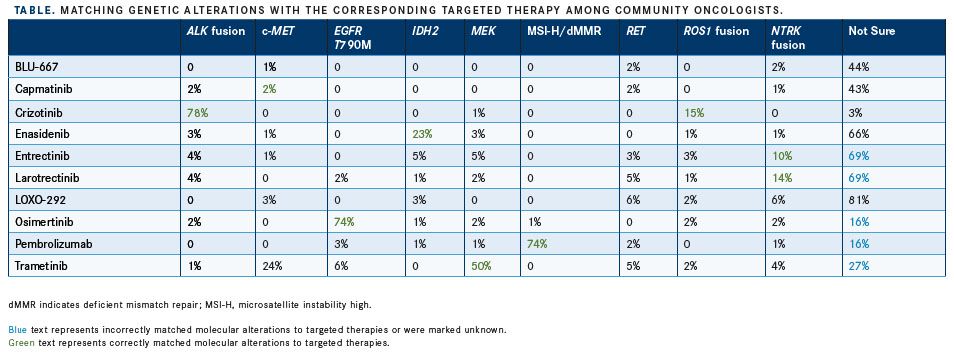
In a data set of 292 oncologists who responded to questions about current awareness and incorporation of molecular testing in the treatment of cancer, almost 69% incorrectly matched the molecular alteration with the appropriate targeted therapy.
NTRKfusions. A second data set was used to compare community-based oncologists with academic-based oncologists, with a focus on individual practice patterns and knowledge about molecular profiling. Findings were presented during a poster session at the 2019 American Society of Clinical Oncology Annual Meeting.1
“We wanted to get a sense of practice patterns,” Bhavana Singh, MD, MSc, lead author, toldTargeted Therapies in Oncologyduring an interview. “It started as a study focused on understanding geographic variation in practice patterns, but evolved into a commentary on [the differences] between community-based oncologists versus academic-based oncologists.”
Community oncologists were polled during 6 case-based research events in 2018, with survey questions focused on awareness of molecular profiling, timing of the testing, and connecting molecular targets to specific therapies. The oncologists were asked to match a genetic alteration to its corresponding targeted therapyinformation that is key to effective personalized treatment. Data for academic oncologists (n = 59) were obtained from a chart review at Georgetown Lombardi Comprehensive Cancer Center. Questions in this data set explored the timing and extent of molecular profiling in disease-specific academic practices (lung, breast, and colorectal cancers).
“Some of the biggest concerns that community oncologists voiced were the cost of molecular profiling and the utility of the results,” said Singh, an oncology fellow at MedStar Georgetown University Hospital working with Lombardi Comprehensive Cancer Center faculty, both institutions in Washington, DC. Those challenges, as well as insurance coverage, remain significant barriers to ordering next-generation sequencing tests, the investigators wrote. “I think [lack of insurance coverage] makes clinicians hesitant to order these tests in the first place because they are concerned that their patient will get stuck with a huge bill at the end of the day.”
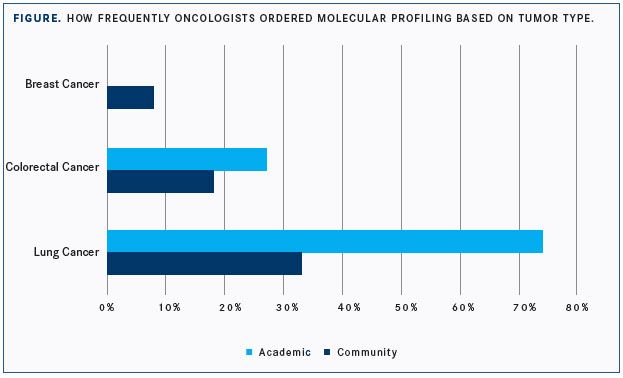
In the community-based cohort, 47% of practices were physician-owned and 41% were hospital-based. Singh et al noted that the frequency at which oncologists ordered molecular profiling varied depending on tumor type, with 33% ordering testing in lung cancer, 18% in colorectal cancer, and 8% in breast cancer. Among oncologists in the academic cohort, 74% ordered molecular profiling for lung cancer, 27% for colorectal cancer, and 0% for breast cancer (FIGURE).
Singh attributed part of the disparity between the community and academic cohorts to how quickly the field of precision oncology is advancing and changing; adding to the complexity are recent FDA approvals for different targeted therapies. “I think in the academic sphere, we take for granted that there’s a lot of buzz around new research findings, but for a community oncologist, it may be a challenge to keep up with what is the latest and greatest,” Singh said.
When oncologists were asked to match the molecular alteration with the appropriate therapy, the high degree of unfamiliarity with some of the newer approved therapies was most telling, said Singh. “To be honest, I actually had to go back and look at some of the targeted agents,” she said. The investigators cited the example of larotrectinib andNTRKfusion (CHART). The FDA approved the agent on November 26, 2018, for adult and pediatric patients with solid tumors that have anNTRKgene fusion without a known acquired resistance mutation, for whom either metastases exist or surgical resection is likely to result in severe morbidity, and who have no satisfactory alternative treatments or whose cancer has progressed following treatment.2Singh did point out, however, that for questions aboutNTRK, the investigators went back to determine when the approval occurred, since the survey questions might have been fielded before the approval.
“I don’t know how fair of an assertion we can make based on this,” Singh said in a release.3“This is a significant knowledge and practice gap, not an issue of negligent treatment.3 Two comprehensive molecular profiling tests now on the market were approved by the FDA in late 2017. Both test for up to 500 genetic mutations, deletions, copy number variations, and rearrangements for which targeted and immune therapies are approved or are under testing and expected to be approved.3
Singh said that most molecular profiling tests provide a good synthesis of the findings. “For example, the report will say that the patient has anNTRKfusion, but underneath that it will provide information about the newer [FDA-approved] drugs that are available and can even suggest chemotherapy combinations that have been shown to work in certain types of malignancies.” But the tests are not always highly specific and can lack clinical applicability, she cautioned.
To address the existing knowledge gap, the investigators argued for additional focused educational activities that facilitate improved knowledge of molecular profiling and corresponding personalized therapeutic strategies.
References:
- Singh BP, Britton SL, Prins P, et al. Molecular profiling (MP) for malignancies: knowledge gaps and variable practice patterns among United States oncologists (Onc) [ASCO abstract 10510). J Clin Oncol. 2019;37(suppl; abstr 10510). ascopubs.org/doi/abs/10.1200/JCO.2019.37.15_suppl.10510.
- FDA approves larotrectinib for solid tumors with NTRK gene fusions. FDA website. www.fda.gov/drugs/fda-approves-larotrectinib-solid-tumors-ntrk-gene-fusions-0. Published December 14, 2018. Accessed June 11, 2019.
- Significant ‘knowledge gap’ exists in use of genetic testing to decide cancer treatment [news release]. Chicago, IL: Georgetown Lombardi Comprehensive Cancer Center; May 30, 2019. lombardi.georgetown.edu/news/Significant-Knowledge-Gap-Exists-in-Use-of-Genetic-Testing-to-Decide-Cancer-Treatment. June 11, 2019.

Powell Reviews Updated IO/TKI Data and AE Management in Endometrial Cancer
April 18th 2024During a Case-Based Roundtable® event, Matthew A. Powell, MD, discussed the case of a patient with advanced endometrial cancer treated with lenvatinib plus pembrolizumab who experienced grade 2 treatment-related hypertension.
Read More
Savona Discusses First-Line JAK Inhibition for Patients With Myelofibrosis at Risk of Anemia
April 17th 2024During a Case-Based Roundtable® event, Michael Savona, MD, and participants discussed the case of a patient with myelofibrosis and moderate anemia receiving JAK inhibitor therapy.
Read More