Treatment Advancements in NSCLC Change Therapy Recommendations in 2019 NCCN Guidelines
Due to an active research landscape, the National Comprehensive Cancer Network Guidelines for non–small cell lung cancer have had 3 recent updates that include numerous clinically relevant recommendations.
Due to an active research landscape, the National Comprehensive Cancer Network (NCCN) Guidelines for nonsmall cell lung cancer (NSCLC) have had 3 recent updates that include numerous clinically relevant recommendations.1
“These new changes to the NCCN guidelines express the rapidly evolving deluge of new data regarding the targeted and immunologic treatment of lung cancer,” said Wallace L. Akerley, MD, senior director of community oncology research at the Huntsman Cancer Institute and professor of internal medicine at The University of Utah in Salt Lake City.
According to Akerley, a guidelines panel expert, the NCCN has kept pace with accelerating research and associated trends. “The NCCN lung panel foreshadowed the current treatment paradigm when it made early recommendations for molecular panel testing for NSCLC and completed the transition to true precision medicine in late 2016 when it dropped the recommendation for erlotinib [Tarceva] for lung cancers without anEGFRmutation,” he said.
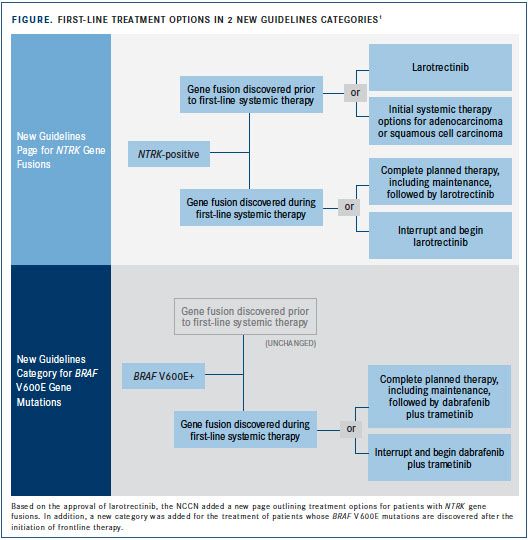
As the past 3 guideline updates demonstrated, molecular medicine is redefining lung cancer diagnosis and treatment. New mutation categories have been added, such as forNTRKgene fusions (FIGURE), and existing biomarkers have been revised, as with the new definition of PD-L1 expression positivity. The latest recommendations also make agents available to more patients and affect treatment sequencing.
“Recently, brigatinib [Alunbrig], osimertinib [Tagrisso], and lorlatinib [Lorbrena] have been added as recommendations for those withALK,EGFR, andROS1mutations in various lines of treatment and combined chemoimmunotherapy for those without a targetable mutation,” Akerley said. “The treatment of NSCLC has been completely overhauled in the last 2-plus years, and the NCCN has been the leader in interpreting these data.”
Guideline updates regularly promise better patient outcomes and offer concise therapy recommendations for busy clinicians who need to stay informed. This review provides an outline of the most recent updates, with brief summaries of relevant trials and FDA approvals.
Alectinib
After showing superiority over crizotinib (Xalkori) in the global Ph3 ALEX study, alectinib (Alecensa) was added as a preferred agent for patients withALKrearrangements discovered during first-line systemic therapy. It is recommended that patients either complete their prescribed regimen or interrupt and initiate treatment with alectinib. Updated results of the Ph3 ALEX trial showed that participants with stage IIIB or IVALK-positive disease treated with alectinib had a median progression-free survival (PFS) of 34.8 months versus 10.9 months for those receiving crizotinib (HR 0.47; 95% CI, 0.34- 0.65;P<.001).2
Brigatinib
In another ALK inhibitor superiority trial, brigatinib demonstrated better efficacy than crizotinib in 275 patients with stage IIIB or IVALK-positive NSCLC.3The confirmed overall response rate (ORR) in patients taking brigatinib was 71% versus 60% in patients taking crizotinib (P= .0678). Of note, brigatinib offered a higher confirmed ORR than crizotinib for patients with measurable intracranial lesions (78% vs 29%;P= .0028). The NCCN now recommends brigatinib as a category 1 option forALK-positive disease discovered prior to first-line systemic therapy or as an alternative option if the mutation is discovered during first-line systemic therapy.
Lorlatinib
Lorlatinib is a third-generation oral TKI active againstALK- andROS1-positive NSCLC. In a phase II trial, a subgroup of 215 patients withALK-positive disease who had received 1 or more previous ALK kinase inhibitors had an ORR of 48%.4Of these patients, 44% were partial responders and 4% had a complete response. Estimated median response duration was 12.5 months, and 60% of patients with measurable intracranial lesions responded to therapy. Based on these findings, lorlatinib received an FDA approval, which prompted new NCCN recommendations. For patients withALK-positive metastatic NSCLC, lorlatinib is now recommended after disease progression on crizotinib, ceritinib (Zykadia), brigatinib, or alectinib; it may also be used after subsequent therapy and progression on the 3 latter agents.
The guidelines also changed forROS1-positive disease for which lorlatinib is now recommended as subsequent therapy for patients withROS1-positive metastatic NSCLC who have progressed after treatment with crizotinib or ceritinib. This indication, however, has yet to receive FDA approval.
Dabrafenib Plus Trametinib
The updated guidelines include a new category for patients that test positive forBRAF V600Emutations and recommend a combination of dabrafenib (Tafinlar) plus trametinib (Mekinist), which inhibit some mutated forms of BRAF and MEK 1/2 kinases, respectively. This recommendation is supported by an FDA approval granted in May 2018 based on results of the phase III COMBI-AD trial.5Patients receiving the combination experienced relapses at a rate of 38% compared with 57% in the placebo arm (HR 0.47; 95% CI, 0.39-0.58;P<.0001).
Dacomitinib
Oral tyrosine kinase inhibitor (TKI) dacomitinib (Vizimpro) was added as a category 1 option for first-line therapy in patients withEGFR-mutationpositive, metastatic NSCLC and is also an option for asymptomatic patients and those with brain or isolated lesions. Dacomitinib received first-line FDA approval on September 27, 2018, following the phase III ARCHER 1050 trial, in which the agent showed better efficacy data than gefitinib (Iressa).6Median PFS for patients with metastatic NSCLC withEGFRexon 19 deletions or exon 21 L858R substitution mutations treated with dacomitinib was 14.7 months compared with 9.2 months for those treated with gefitinib (P<.0001).
Durvalumab
Durvalumab (Imfinzi), a checkpoint inhibitor that prevents PD-L1 from binding with PD-1 and CD80, showed promise in new data from the phase III PACIFIC trial in 2017 and 2018. In total, 713 patients with NSCLC and no disease progression following chemoradiotherapy were randomized 2:1 to receive either durvalumab or placebo.7Recent results showed that durvalumab extended PFS by a considerable margin, with a median duration of 17.2 versus 5.6 months (P<.001). The 2-year OS rate was 66.3% with durvalumab compared with 55.6% in the placebo group (P= .005). Median time to distant metastasis or death was also improved (28.3 vs 16.2 months). Durvalumab received FDA approval on February 16, 2018, following release of initial PFS results in 2017.8Based on the results from PACIFIC, the NCCN changed the recommendation level for durvalumab from category 2A to category 1. Specifically, durvalumab is now recommended for consolidation therapy in patients with unresectable stage III NSCLC who have not had disease progression after 2 or more cycles of concurrent platinum-based chemotherapy.
Larotrectinib
On November 26, 2018, larotrectinib (Vitrakvi) received FDA approval for patients with solid tumors andNTRKgene fusions without a known acquired resistance mutation or metastatic or unresectable disease and who have no satisfactory alternative therapy. Because larotrectinib is effective across ages and tumor types, it is considered an age- and tumor-agnostic therapy.9Three multicenter clinical trialsSCOUT, NAVIGATE, and LOXO-TRK-14001—evaluated larotrectinib for a variety of cancer types, prompting FDA approval and NCCN recommendation. Out of 55 patients in the trials with unresectable or metastatic tumors, ORR was 75%, divided between partial and complete responders at rates of 53% and 22%, respectively. A recent data update showed that 1-year OS was 90%, with 69% still responding and slightly more than half of the patients (58%) remaining progression free.10Based on the FDA approval and these results, the NCCN now recommends larotrectinib as first-line therapy for patients withNTRKgene fusion positive metastatic NSCLC. In addition, the guidelines now include an entire page diagramming the treatment of patients withNTRKaberrations.
Osimertinib
Another oral TKI, osimertinib, inhibitsEGFRsensitizing mutations and the T790M resistance mutation which occurs in about 60% of patients after first-line TKI therapy. Based on results from the phase III FLAURA trial, the NCCN now considers osimertinib the preferred first-line agent inEGFRmutation positive NSCLC (category 1 recommendation).11The trial involving 556 patients with treatment-naïve,EGFRmutation-positive advanced NSCLC showed that osimertinib produced a median PFS of 18.9 months versus 10.2 months with standard gefitinib or erlotinib (HR, 0.46; 95% CI, 0.37-0.57;P<.001). ORR was comparable between osimertinib and standard EGFR TKIs, at 80% and 76%, respectively (odds ratio, 1.27; 95% CI, 0.85 to 1.90;P= .24). At 18 months, 83% of the patients in the osimertinib group were still alive compared with 71% of patients receiving standard therapy. Fewer patients receiving osimertinib had grade ≥3 adverse events than those receiving standard EGFR TKIs (35% vs 45%), which further supports the superiority of osimertinib.
Patients With PD-L1 >50%
To go along with updates in immunotherapy and chemotherapy combinations in 2018, the NCCN changed PD-L1 testing from a category 2A recommendation to category 1 for both squamous and nonsquamous disease. Eligibility for checkpoint inhibitor therapy was clarified to include patients with PD-L1 expression ≥50% rather than “PD-L1 positivity,” per the old guidelines. For patients within these criteria who do not harborEGFRorALKabnormalities, 3 chemotherapy and immunotherapy agent combinations joined the checkpoint inhibitor pembrolizumab (Keytruda) on the list of recommended frontline therapies:
For patients with nonsquamous NSCLC, a combination of carboplatin or cisplatin plus pemetrexed and pembrolizumab received an FDA approval on August 20, 2018.12This approval was based on results from the phase III KEYNOTE-189 trial in which 616 treatment-naïve patients with metastatic disease were randomized to chemotherapy plus pembrolizumab or placebo. Patients in the pembrolizumab arm had a significantly higher ORR (48% vs 19%;P= .0001), overall survival (OS; HR 0.49; 95% CI, 0.38-0.64;P<.00001), longer median PFS (8.8 vs 4.9 months), and longer median response duration (11.2 vs 7.8 months).
Also for patients with nonsquamous NSCLC, the combination of carboplatin, paclitaxel, bevacizumab (Avastin), and atezolizumab (Tecentriq) received FDA approval on December 6, 2018, following results of the phase III IMpower150 trial, in which 1202 patients with metastatic nonsquamous NSCLC were randomized to 3 study arms, all including a duo of carboplatin and paclitaxel.13The checkpoint inhibitor atezolizumab and VEGF inhibitor bevacizumab were added individually and then together to the chemotherapy duo, with the full 4-drug regimen ultimately demonstrating superiority. Results showed a median OS of 19.2 months for patients on the 4-drug regimen compared with those in the control group, who had an OS of 14.7 months when receiving carboplatin, paclitaxel, and bevacizumab (P = .016). Similar gains were seen in median PFS (8.5 vs 7.0 months;P= .0002) and ORR (55% vs 42%). Of note, 36% of trial participants developed antidrug antibodies to atezolizumab. Although survival was similar regardless of antidrug status, future atezolizumab studies will monitor for any impact on efficacy, safety, and pharmacokinetics.
On October 30, 2018, a combination of carboplatin or cisplatin and either paclitaxel or nab-paclitaxel (Abraxane) with pembrolizumab received FDA approval for patients with squamous NSCLC. This approval was supported by the KEYNOTE-407 trial, in which 559 patients were randomized to receive pembrolizumab or placebo with chemotherapy.14Like the 2 previously mentioned studies, adding the checkpoint inhibitor demonstrated significant advantages. Along with a better median OS (15.9 vs 11.3 months;P= .0017), patients receiving pembrolizumab had an improved median PFS (6.4 vs 4.8 months;P< .0001), ORR (58% vs 35%;P= .0008), and estimated median response duration (7.2 vs 4.9 months).
References:
- NCCN Clinical Practice Guidelines in Oncology. Non-Small Cell Lung Cancer, version 3.2019. National Comprehensive Cancer Network website. nccn.org/professionals/physician_gls/pdf/nscl.pdf. Published January 18, 2019. Accessed February 13, 2019.
- Peter S, Camidge DR, Shaw AT, et al. Alectinib versus Crizotinib in Untreated ALK-Positive NonSmall Cell Lung Cancer. N Engl J Med. 2017;377(9):829–838. doi: 10.1056/NEJMoa1704795.
- FDA approves lorlatinib for second- or third-line treatment of ALK-positive metastatic NSCLC. FDA website. www.fda.gov/Drugs/InformationOnDrugs/ApprovedDrugs/ucm625027.htm. Published December 17, 2018. Accessed February 20, 2019.
- Camidge R, Kim HR, Ahn M, et al. Brigatinib vs crizotinib in patients with ALK inhibitor-naïve advanced ALK+ NSCLC: first report of a phase 3 trial (ALTA-1L). Presented at: International Association for the Study of Lung Cancer 19th World Conference on Lung Cancer; September 23-26, 2018; Toronto, Ontario, Canada. Abstract PL02.03. jto.org/article/S1556-0864(18)30969-9/abstract.
- FDA approves dabrafenib plus tremetinib for adjuvant treatment of melanoma with BRAF V600E or V600K mutations. FDA website. www.fda.gov/Drugs/InformationOnDrugs/ApprovedDrugs/ucm606165.htm. Updated May 1, 2018. Accessed February 20, 2019.
- FDA approves dacomitinib for metastatic nonsmall cell lung cancer. FDA website. www.fda.gov/Drugs/InformationOnDrugs/ApprovedDrugs/ucm621967.htm. Updated December 17, 2018. Accessed February 20, 2019.
- Antonia SJ, Villegas A, Daniel D, et al. PACIFIC Investigators. Overall survival with durvalumab after chemoradiotherapy in stage III NSCLC.N Engl J Med.2018;379(24):2342-2350. doi: 10.1056/NEJMoa1809697.
- FDA approves durvalumab after chemoradition for unresectable stage III NSCLC. FDA website. www.fda.gov/Drugs/InformationOnDrugs/ApprovedDrugs/ucm597248.htm. Updated February 20, 2018. Accessed February 20, 2019.
- FDA approves larotrectinib for solid tumors with NTRK gene fusions. FDA website. www.fda.gov/Drugs/InformationOnDrugs/ApprovedDrugs/ucm626720.htm. Updated December 17, 2018. Accessed February 20, 2019.
- Lassen UN, Albert CM, Kummar S, et al. Larotrectinib efficacy and safety in TRK fusion cancer: an expanded clinical dataset showing consistency in an age and tumor agnostic approach. Presented at: ESMO 2018 Congress; October 19-23, 2018; Munich, Germany. Abstract 4090. doi.org/10.1093/annonc/mdy279.397.
- Soria JC, Yuichiro O, Vansteenkiste J, et al; FLAURA investigators. Osimertinib in untreated EGFR-mutated advanced non-small-cell lung cancer.N Engl J Med.2018;378(2):113-125. doi: 10.1056/NEJMoa1713137.
- FDA grants regular approval for pembrolizumab in combination with chemotherapy for first-line treatment of metastatic nonsquamous NSCLC. FDA website. www.fda.gov/Drugs/InformationOnDrugs/ApprovedDrugs/ucm617471.htm. Updated August 20, 2018. Accessed February 20, 2019.
- Socinski MA, Jotte RM, Cappuzzo F, et al; IMpower150 Study Group. Atezolizumab for First-Line Treatment of Metastatic Nonsquamous NSCLC.N Engl J Med.2018;378(24):2288-2301. doi: 10.1056/NEJMoa1716948.
- FDA approves pembrolizumab in combination with chemothreapy for first-line treatment of metatstatic squamous NSCLC. FDA website. www.fda.gov/Drugs/InformationOnDrugs/ApprovedDrugs/ucm624659.htm. Updated December 17, 2018. Accessed February 20, 2019.
Ornstein Advises on Starting Dose and Management of Lenvatinib in RCC
April 21st 2024During a Case-Based Roundtable® event, Moshe Ornstein, MD, MA, provided guidance on dosing and toxicity concerns in a patient treated with lenvatinib plus pembrolizumab for advanced renal cell carcinoma.
Read More