Pembrolizumab Leads to Promising Outcomes in Advanced ASCC
Pembrolizumab demonstrated positive activity in patients with previously treated advanced anal squamous cell carcinoma, regardless of PD-L1 expression, according to the results of the KEYNOTE-158 trial presented at the 2020 Gastrointestinal Cancers Symposium.

Pembrolizumab (Keytruda) demonstrated positive activity in patients with previously treated advanced anal squamous cell carcinoma (ASCC), regardless of PD-L1 expression, according to the results of the KEYNOTE-158 trial presented at the 2020 Gastrointestinal Cancers Symposium.1
“Pembrolizumab demonstrated antitumor activity, durable response, and encouraging overall survival with manageable safety in patients with previously treated anal squamous cell carcinoma,” Aurélien Marabelle, MD, PhD, a senior medical oncologist at Gustave Roussy in France, said when presenting the results of the phase II trial.
In the prior phase Ib multicohort KEYNOTE-028 trial, pembrolizumab monotherapy demonstrated modest antitumor activity and acceptable safety in patients with advanced anal carcinoma and positive PD-L1 expression. The objective response rate (ORR) was 17% among 24 patients with squamous cell carcinoma histology, and an additional 42% had stable disease. The median duration of response (DOR) was not reached.2
KEYNOTE-158 is an ongoing multicohort phase II trial exploring the efficacy and safety of the antiPD-1 antibody in patients with advanced solid tumors (NCT02628067). The ASCC cohort enrolled patients who had progressed on or had developed an intolerance to ≥1 prior line of therapy in the unresectable and/or metastatic disease setting.1
Patients underwent PD-L1 testing using the PD-L1 IHC 22C3 pharmDx assay, and their expression levels were defined by combined positive score (CPS). PD-L1 expression level was not a criterion of enrollment in the trial.
A total of 112 patients received 200 mg of intravenous pembrolizumab every 3 weeks for up to 35 cycles, or approximately 2 years.
The primary end point was ORR, as assessed by central review using RECIST v1.1 criteria, and secondary end points included DOR, progression-free survival (PFS), OS, and safety.
The median age of all patients was 61 (range, 32-79). A majority of patients had an ECOG performance status of 1 (59.8%), M1 stage disease (92.9%), and positive PD-L1 expression (CPS, ≥1; 67.0%). About three-quarters of the patients in the cohort (74.1%) were being treated beyond the second line, and most had previously received radiation therapy (92.9%). Marabelle noted that human papillomavirus status was not reported for the patients in the study.
A total of 112 patients received 200 mg of intravenous pembrolizumab every 3 weeks for up to 35 cycles, or approximately 2 years. The primary end point was ORR, as assessed by central review using RECIST v1.1 criteria, and secondary end points included DOR, progression-free survival (PFS), OS, and safety. The median age of all patients was 61 (range, 32-79). A majority of patients had an ECOG performance status of 1 (59.8%), M1 stage disease (92.9%), and positive PD-L1 expression (CPS, ≥1; 67.0%). About three-quarters of the patients in the cohort (74.1%) were being treated beyond the second line, and most had previously received radiation therapy (92.9%). Marabelle noted that human papillomavirus status was not reported for the patients in the study.
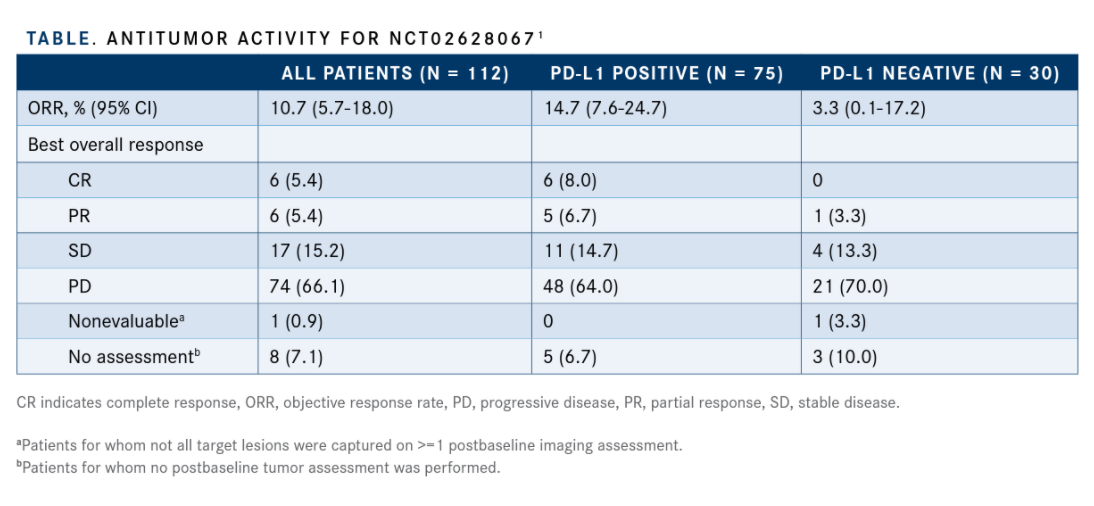
The ORR was 10.7% (95% CI, 5.7%-18.0%) in the overall cohort, with 6 complete responses and 6 partial responses. Seventeen patients (15.2%) also achieved stable disease. However, 9 patients were not included in the evaluation. Among the 75 patients with positive PD-L1 expression, the ORR was 14.7% (95% CI, 7.6%24.7%); the ORR was 3.3% (95% CI, 0.1%-17.2%) in patients with negative expression (TABLE).
The median DOR was not yet reached at the time of data cutoff (range, 6.0+ to 33.9+ months). At both 12 and 24 months, the DOR rate was 90%.
The median PFS was 2.0 months (95% CI, 2.0-2.1), and at 1 year, the PFS rate was 15%. Median OS was 11.9 months (95% CI, 9.1-14.9). At 1 and 2 years, the OS rates were 49.1% and 25.8%, respectively.
Treatment-related adverse events (AEs) were reported in 60.7% of patients, with grade 3/4 events occurring in 17.9%. The most frequent treatment-related AEs included fatigue (15.2%), diarrhea (11.6%), hypothyroidism (11.6%), nausea (11.6%), asthenia (8.9%), and pruritus (7.1%).
Investigators observed immune-mediated AEs and infusion reactions of any grade and grade 3/4 in 23.2% and 4.5% of patients, respectively. The most common immune-mediated AE was hypothyroidism, in 11.6% of patients, followed by hyperthyroidism, in 6.3%. Only 1 patient had a mild infusion reaction.
“These are typical adverse events that are seen with pembrolizumab and antiPD-1 treatments,” Marabelle commented, adding that no differences were observed in the type of incidence of the AEs.”
References
- Marabelle A, Cassier PA, Fakih M, et al. Pembrolizumab for advanced anal squamous cell carcinoma (ASCC): results from the multicohort, phase II KEYNOTE-158 study.J Clin Oncol. 2020;38(suppl 4; abstr 1). doi: 10.1200/JCO.2020.38.4_suppl.1.
- Ott PA, Piha-Paul SA, Munster P, et al. Safety and antitumor activity of the anti-PD-1 antibody pembrolizumab in patients with recurrent carcinoma of the anal canal.Ann Oncol. 2017;28(5):1036-1041. doi: 10.1093/ annonc/mdx029.
Novel Approaches Focus on Limiting Toxicity in Older Patients with ALL
April 22nd 2024The major challenges for clinicians treating older patients with acute lymphoblastic leukemia surround the emergence of resistance to existing therapies and the toxicities associated with current chemotherapies.
Read More
Ornstein Advises on Starting Dose and Management of Lenvatinib in RCC
April 21st 2024During a Case-Based Roundtable® event, Moshe Ornstein, MD, MA, provided guidance on dosing and toxicity concerns in a patient treated with lenvatinib plus pembrolizumab for advanced renal cell carcinoma.
Read More
The Impact of the Gut Microbiome in Young Patients With Colorectal Cancer
February 15th 2021In season 2, episode 2 of Targeted Talks, Cathy Eng, MD, speaks with Benjamin Weinberg, MD, about the gut microbiome, and how the presence of certain microbiota impact the onset and intensity of disease as well as the potential response to certain treatments.
Listen
Peers Discuss Role of Pola-R-CHP vs R-CHOP in Newly Diagnosed DLBCL
April 19th 2024During a Case-Based Roundtable® event, Haifaa Abdulhaq, MD discussed with participants whether the POLARIX trial influences their choice to use the pola-R-CHP as opposed to R-CHOP regimen for patients with newly diagnosed diffuse large B-cell lymphoma.
Read More