ASCO Issues Update on Frontline NSCLC Treatment in Wake of Immunotherapy Approvals
In response to frequent and substantial changes in the treatment of non–small cell lung cancer in the frontline set­ting, the American Society of Clinical Oncology has partially updated its 2017 guide­line for the treatment of patients with stage IV disease without driver mutations.
In response tofrequent and substantial changes in the treatment of nonsmall cell lung cancer (NSCLC) in the frontline set­ting, the American Society of Clinical Oncology (ASCO) has partially updated its 2017 guide­line for the treatment of patients with stage IV disease without driver mutations.1
“Since 2017, additional molecular abnormal­ities have been identified, which necessitated separate guidelines for those with targetable driver mutations and those without targetable mutations,” Nasser H. Hanna, MD, lead author of the guidelines, said in an interview withTargeted Therapies in Oncology. “Several randomized phase III trials evaluating dif­ferent chemotherapy, immunotherapy, and antiangiogenic regimens have been reported. These results required a rewriting of key recommendations [to include] guidance on what the committee believed are the preferred treatments that cliniciansshoulduse and additional regimens that cliniciansmayuse in given circumstances.”
In 2017, prior to the current update, ASCO had last revised the Clinical Practice Guideline on the systemic treatment of patients with stage IV NSCLC.2Of note, 5 randomized con­trolled trials from 2015 to 2019 provided the evidence used to update the recommendations for the care of patients with an ECOG perfor­mance score of 0 to 1 whose tumors do not have a driver alteration inALKorEGFR. These trials investigated the antiPD-1 antibody pembroli­zumab (Keytruda) as monotherapy and in com­bination with chemotherapy (KEYNOTE-042, KEYNOTE-189, and KEYNOTE-407),3-5or eval­uated combinations featuring the PD-L1 inhib­itor atezolizumab (Tecentriq; IMpower150 and IMpower130).6,7
Nonsquamous NSCLC
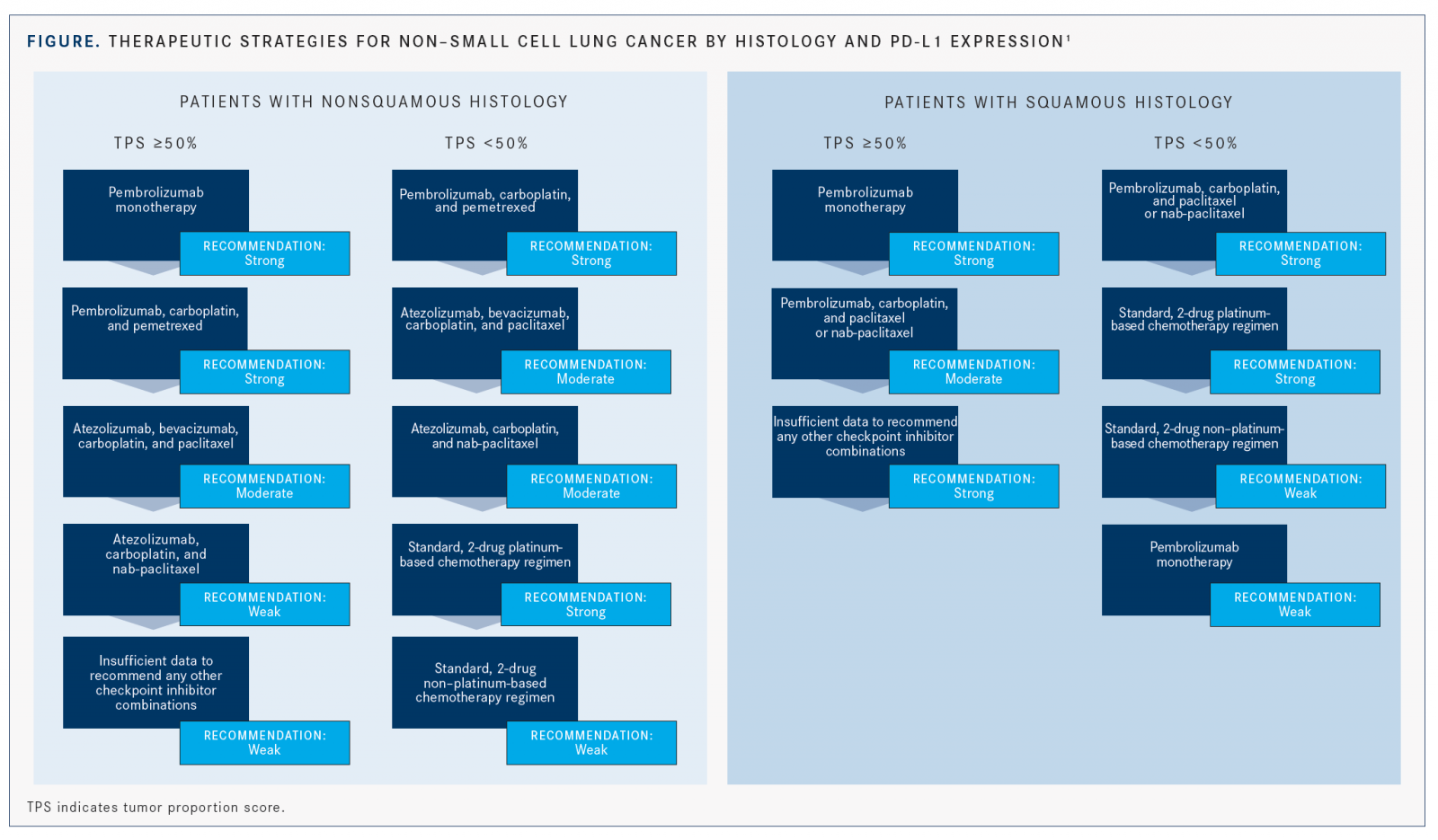
The ASCO recommendations divide patients with nonsquamous histology into 2 main groups: those with high PD-L1 expression (with a tumor proportion score [TPS] ≥50%) and those with either negative (TPS 0%) or low positive PD-L1 expression (TPS between 1% and 49%) (FIGURE).1
High PD-L1 Expression
For patients with TPS ≥50%, the recom­mendation with the most support remains single-agent pembrolizumab, which is con­sistent with the 2017 recommendation.1,2This indication was approved by the FDA in 2016, based on findings from KEYNOTE-024.8Data from KEYNOTE-042 have since been published and further support the use of pembrolizumab monotherapy in this setting.3
The randomized, open-label phase III KEYNOTE-042 trial in patients with both histologies and PD-L1 TPS ≥1% showed that overall survival (OS) was significantly improved with pembrolizumab in those with TPS ≥50% (HR, 0.69; 95% CI, 0.56-0.85;P= .0003).3In the population with nonsquamous NSCLC and TPS ≥50%, pembrolizumab resulted in numerically higher OS but failed to reach statistical signif­icance (HR, 0.82; 95% CI, 0.63-1.07;P= not significant).1 Grade ≥3 adverse events (AEs) for all patients with TPS ≥1% favored the interven­tion, at 17.8% versus 41% with the control.1
In this study, patients receiving single-agent pembrolizumab had superior survival compared with a platinum-based doublet regimen,” said Nasser, who is also a Tom and Julie Wood Family Foundation Professor of Lung Cancer Clinical Research at Indiana University School of Med­icine in Indianapolis. When asked about the OS results of this trial, he added that the study was not powered to show a survival advantage in this subset; therefore, [the OS benefit] was not statistically significant. However, it was clear and convincing that this subset contributed the most to the OS advantage.”
Additionally, patients with TPS ≥ 50% and nonsquamous histology may be offered pem­brolizumab plus carboplatin and pemetrexed (Alimta). The guideline suggests that clinicians take a shared decision-making approach with patients to determine whether the single agent or the combination is a more suitable option because a lack of randomized trial data comparing the 2 may lead to difficulty in treatment selection.
The recommendation is supported by the results of the double-blind, phase III KEYNOTE-189 trial, which examined the immunochemotherapy combination versus chemotherapy alone in all patients with non­squamous NSCLC. One-year OS was signifi­cantly improved in all the PD-L1 subgroups, including the TPS ≥50% group (HR, 0.42; 95% CI, 0.26-0.68), and rates of AEs were similar in both arms.4
Another immunotherapy-and-chemotherapy combination that may be employed to treat this patient population is the 4-drug regimen of atezolizumab, bevacizumab (Avastin), carbopla­tin, and paclitaxel. The phase III IMpower150 trial demonstrated a clinically meaningful and statistically significant improvement in pro­gression-free survival (PFS) in patients treated with the quadruplet regimen versus bevacizum­ab plus chemotherapy alone (HR, 0.39; 95% CI, 0.25-0.60 for patients with high PD-L1 expres­sion).5 OS for these patients was also improved per an updated analysis (HR, 0.70; 95% CI, 0.43-1.13).9 Grade 3/4 AEs were higher in the 4-drug arm.1
“The data with combination immunother­apy plus VEGF inhibition [are] compelling,” said Nasser. “The 1-year PFS [rate] with the IMpower150 regimen is actually the best 1-year PFS rate of the studies reported in this patient population.”
Patients for whom this 4-drug regimen may be considered instead of pembrolizumab include those with high symptom or disease burden or large-volume visceral tumor involve­ment. The increased toxicity and higher price of the atezolizumab/bevacizumab/carboplatin/ paclitaxel combination should be carefully considered when choosing between this ther­apy and pembrolizumab. The guideline also points out that the IMpower150 investigators did not report the results for the compara­tor arm of atezolizumab plus chemotherapy. Because a comparison of treatment with and without bevacizumab has not been described, the benefit of adding the VEGF inhibitor remains unclear.1
Finally, patients with TPS ≥ 50% may also receive atezolizumab, carboplatin, and nab-pa­clitaxel (Abraxane), according to results of the IMpower130 trial, which showed a slight numerical OS advantage of the combination ver­sus chemotherapy alone (17.3 months vs 16.9 months; HR, 0.84; 95% CI, 0.51-1.39).6
The recommendations for this patient group conclude by stating that there are insufficient data to recommend other single-agent immune checkpoint inhibitors, immunotherapy combinations, or immunotherapy-chemother­apy combinations for the first-line treatment of this group.1
Low/Negative PD-L1 Expression
For patients with unknown, low positive, or negative PD-L1 expression and nonsquamous disease, the regimen with the most support per the guideline update is the KEYNOTE-189 combination of pembrolizumab plus carbopla­tin and pemetrexed.1,4In that trial’s subgroup of patients with low positive PD-L1 expression (TPS between 1% and 49%), the 12-month OS (HR, 0.55; 95% CI, 0.34-0.90) and PFS (HR, 0.55; 95% CI, 0.37-0.81) were significantly improved with the addition of pembrolizumab.1,4 Improve­ments in OS (HR, 0.59; 95% CI, 0.38-0.92) and PFS (HR, 0.75; 95% CI, 0.53-1.05) were also observed in the cohort of patients with negative PD-L1 expression (TPS <1%).1,4
Patients in this group may also receive the quadruplet from the IMpower150 trial: beva­cizumab, atezolizumab, carboplatin, and pacli­taxel. Patients considered to have low positive tumor PD-L1 expression in that trial showed a statistically significant improvement in PFS, which was 8.3 months versus 6.6 months in the non-atezolizumab arm, although there was not a large clinical difference (HR, 0.56; 95% CI, 0.41- 0.77).5Similarly, a subset analysis of patients with PD-L1negative tumors failed to show a clini­cally meaningful improvement in PFS (7.1 months vs 6.9 months), although it did reach statistical significance (P= .039).1In the updated analysis, OS improvements were seen with the atezolizum­ab regimen versus the control in all subgroups including PD-L1low and PD-L1–negative tumors (HR, 0.80; 95% CI, 0.55-1.15 and HR, 0.82; 95% CI, 0.62-1.08, respectively).9
Alternatively, patients can receive atezoli­zumab, carboplatin, and nab-paclitaxel. In patients with PD-L1 expression between 1% and 49% in the IMpower130 trial, median OS was 23.7 months for the atezolizumab group versus 15.9 months with chemotherapy alone, which was not statistically significant (HR, 0.70; 95% CI, 0.45-1.08). Median PFS in the same subgroup was 8.3 months versus 6.0 months (HR, 0.61; 95% CI, 0.43-0.85).6
Finally, patients with contraindications to immunotherapy or who decline it should be treated with standard 2-drug, platinum-based chemotherapy, and those who are also not suit­able for platinum-based therapy should receive nonplatinum-containing regimens. These rec­ommendations remain unchanged from previ­ous guidelines.1,2As a last resort, single-agent pembrolizumab may be offered to patients with low positive PD-L1 expression who are ineligible for platinum doublet therapy with or without pembrolizumab.1
“I would estimate that 10% to 20% of patients have a contraindication to immunotherapy and should be offered chemotherapy alone,” Nasser said. “Those with contraindications to immunotherapy include patients with severe autoimmune disorders such as Crohn disease, patients with transplanted organs on chronic immunosuppression, and patients who require high-dose steroids for other medical indica­tions, including severe chronic obstructive pulmonary disorder. There are also patients with interstitial lung disease who should not receive immunotherapy.”
Squamous NSCLC
As with patients with nonsquamous histology, the updated treatment guideline classifies those with squamous NSCLC according to tumor PD-L1 expression level: high (TPS ≥50%) versus negative (TPS 0%) or low positive (TPS 1% to 49%).
High PD-L1 Expression
Based on the same evidence outlined in patients with nonsquamous histology as mentioned earlier and in line with the previous guideline, those with squamous NSCLC and TPS ≥50% should receive single-agent pembrolizumab.1,2
Data from KEYNOTE-042 further support this recommendation, with a statistically significant improvement in OS observed with the PD-1 inhibitor monotherapy in those with squamous histology and TPS ≥50 (HR, 0.53; 95% CI, 0.38-0.75).3Similarly, median PFS was statistically significantly greater with pembrolizumab, although the prespecified superiority boundary was not met.1
Clinicians may also offer pembrolizumab plus carboplatin and either paclitaxel or nab-paclitaxel based on evidence from the KEYNOTE-407 trial.1The results of this trial led to the approval of the combination in all patients, regardless of PD-L1 status in the first line in 2018.10 In the subgroup analysis of patients with PD-L1 TPS ≥50%, the hazard ratio for OS was 0.64 (95% CI, 0.37-1.10), and a statistically significant improvement in median PFS was observed (8.0 months vs 4.2 months; HR, 0.37; 95% CI, 0.24-0.58).7
According to the guideline, data supporting the use of other immune checkpoint inhibitors, immunotherapy combinations, or immunother­apy-chemotherapy combinations in the front line are insufficient.1
Low/Negative PD-L1 Expression
For patients with squamous NSCLC and negative or low positive PD-L1 expression, the regimen with the best supportive evidence is pembroli­zumab plus carboplatin and either paclitaxel or nab-paclitaxel, which is also supported by the KEYNOTE-407 trial.1
In the subgroup of patients with negative or low positive PD-L1 expression on the trial, the estimated rates of 1-year OS were improved in the TPS <1% group (64.2% vs 43.3%; HR, 0.61; 95% CI, 0.38-0.98) and the TPS 1%-to-49% group (65.9% vs 50.0%; HR, 0.57; 95% CI, 0.36-0.90).7
For patients in this population who have contra­indications to immunotherapy, the guideline rec­ommends a 2-drug platinum-based chemotherapy regimen; a nonplatinum-based chemotherapy doublet is recommended for patients who are also not candidates for platinum-based therapy.1
Finally, patients with low positive PD-L1 expression who are ineligible for or who decline platinum doublet/pembrolizumab combination treatment may be offered single-agent pem­brolizumab in the absence of contraindications, based on data from the KEYNOTE-042 trial reviewed previously.1
Nasser concluded by pointing out the sig­nificant degree of changes in this update to the guideline.
“The changes [between] the 2017 and 2020 guidelines are substantial,” he said. “Therefore, this is the first time we will have a separate guideline for those without targetable driver mutations.”
References
- Hanna NH, Schneider BJ, Temin S, et al. Therapy for stage IV non-small-cell lung cancer without driver alterations: ASCO and OH (CCO) Joint Guideline Update [published online January 28, 2020]. J Clin Oncol. doi: 10.1200/JCO.19.03022.
- Hanna NH, Johnson D, Temin S, et al. Systemic therapy for stage IV nonsmall-cell lung cancer: American Society of Clinical Oncology Clin­ical Practice Guideline Update.J Clin Oncol.2017;35(30):3484-3515. doi: 10.1200/JCO.2017.74.6065.
- Mok TS, Wu Y-L, Kudaba I, et al; KEYNOTE-042 Investigators. Pem­brolizumab versus chemotherapy for previously untreated, PD-L1 ex­pressing, locally advanced or metastatic non-small-cell lung cancer (KEY­NOTE-042): a randomised, open-label, controlled, phase 3 trial.Lancet.2019;393(10183):1819-1830. doi: 10.1016/S0140-6736(18)32409-7.
- Gandhi L, Rodríguez-Abreu D, Gadgeel S, et al; KEYNOTE-189 Inves­tigators. Pembrolizumab plus chemotherapy in metastatic nonsmall-cell lung cancer.N Engl J Med.2018;378(22):2078-2092. doi: 10.1056/ NEJMoa1801005.
- Socinski MA, Jotte RM, Cappuzzo F, et al; IMpower150 Study Group. Atezolizumab for first-line treatment of metastatic nonsquamous NSCLC.N Engl J Med.2018;378(24):2288-2301. doi: 10.1056/NEJMoa1716948.
- Paz-Ares L, Luft A, Vincente D, et al; KEYNOTE-407 Investiga­tors. Pembrolizumab plus chemotherapy for squamous nonsmall-cell lung cancer.N Engl J Med.2018;379(21):2040-2051. doi: 10.1056/ NEJMoa1810865.
- West H, McCleod M, Hussein M, et al. Atezolizumab in com­bination with carboplatin plus nab-paclitaxel chemotherapy com­pared with chemotherapy alone as first-line treatment for metastatic non-squamous non-small-cell lung cancer (IMpower130): a multicentre, randomised, open-label, phase 3 trial.Lancet Oncol.2019;20(7):924-937. doi: 10.1016/S1470-2045(19)30167-6.
- FDA approves Merck’s Keytruda (pembrolizumab) in metastatic NSCLC for the first-line treatment of patients whose tumors have high PD-L1 expression (tumor proportion score [TPS] of 50 percent or more) with no EGFR or ALK genomic tumor aberrations [news release]. Kenilworth, NJ: Merck; October 24, 2016. Merck website. bit.ly/2uIvcgG. Accessed February 13, 2020.
- Socinski MA, Jotte RM, Cappuzzo F, et al. Overall survival (OS) anal­ysis of IMpower150, a randomized Ph 3 study of atezolizumab (atezo) + chemotherapy (chemo) ± _bevacizumab (bev) vs chemo + bev in 1L nonsquamous (NSQ) NSCLC.J Clin Oncol.2018;36(suppl; abstr 9002). doi: 10.1200/JCO.2018.36.15_suppl.9002.
- FDA approves pembrolizumab in combination with chemotherapy for first-line treatment of metastatic squamous NSCLC. FDA website. bit. ly/3bxMCNv. Published October 30, 2018. Accessed February 13, 2020.

Powell Reviews Updated IO/TKI Data and AE Management in Endometrial Cancer
April 18th 2024During a Case-Based Roundtable® event, Matthew A. Powell, MD, discussed the case of a patient with advanced endometrial cancer treated with lenvatinib plus pembrolizumab who experienced grade 2 treatment-related hypertension.
Read More
Savona Discusses First-Line JAK Inhibition for Patients With Myelofibrosis at Risk of Anemia
April 17th 2024During a Case-Based Roundtable® event, Michael Savona, MD, and participants discussed the case of a patient with myelofibrosis and moderate anemia receiving JAK inhibitor therapy.
Read More
Biomarker Testing Paves the Way for Better Targeted Therapies in NSCLC
April 16th 2024At a live virtual event, Edward S. Kim, MD, MBA, discussed the evolving landscape of biomarker testing before making treatment decisions for patients with early-stage non–small cell lung cancer (NSCLC).
Read More