Before Starting Targeted Therapy for Prostate Cancer, Determine Cardiovascular Risk
Elderly patients with advanced prostate cancer should be evaluated for preexisting cardiovascular diseases before taking oral androgen signaling inhibitors by a multidisciplinary team, includ­ing a cardiologist, according to a recent retro­spective study. Investigators at Thomas Jeffer­son University in Philadelphia, Pennsylvania, demonstrated that after receiving abiraterone acetate or enzalutamide, these patients had higher rates of short-term mortality than similar patients without CVDs.

Elderly patients withadvanced prostate cancer should be evaluated for preexisting cardiovascular diseases (CVDs) before taking oral androgen signaling inhibitors by a multidisciplinary team, includ­ing a cardiologist, according to a recent retro­spective study. Investigators at Thomas Jeffer­son University in Philadelphia, Pennsylvania, demonstrated that after receiving abiraterone acetate (Zytiga) or enzalutamide (Xtandi), these patients had higher rates of short-term mortality than similar patients without CVDs.1
“Once clinical trials demonstrate the efficacy of a drug in a selective group of patients, the next step is to study whether the drug is effective in the population excluded from the pivotal trials,” lead author Grace Lu-Yao, PhD, MPH, associate director of population science at the Sidney
Kimmel Cancer CenterJefferson Health, wrote in an email interview withTargeted Therapies in Oncology.
“It is common that patients not eligible for the pivotal trials receive the approved drugs,” she continued. “The benefits observed in clini­cal trials may not apply to patients who do not meet the trial eligibility criteria. In fact, it has been shown that the net risks may outweigh the benefits for patients treated with androgen-deprivation therapy [ADT] if they have signifi­cant preexisting conditions.”
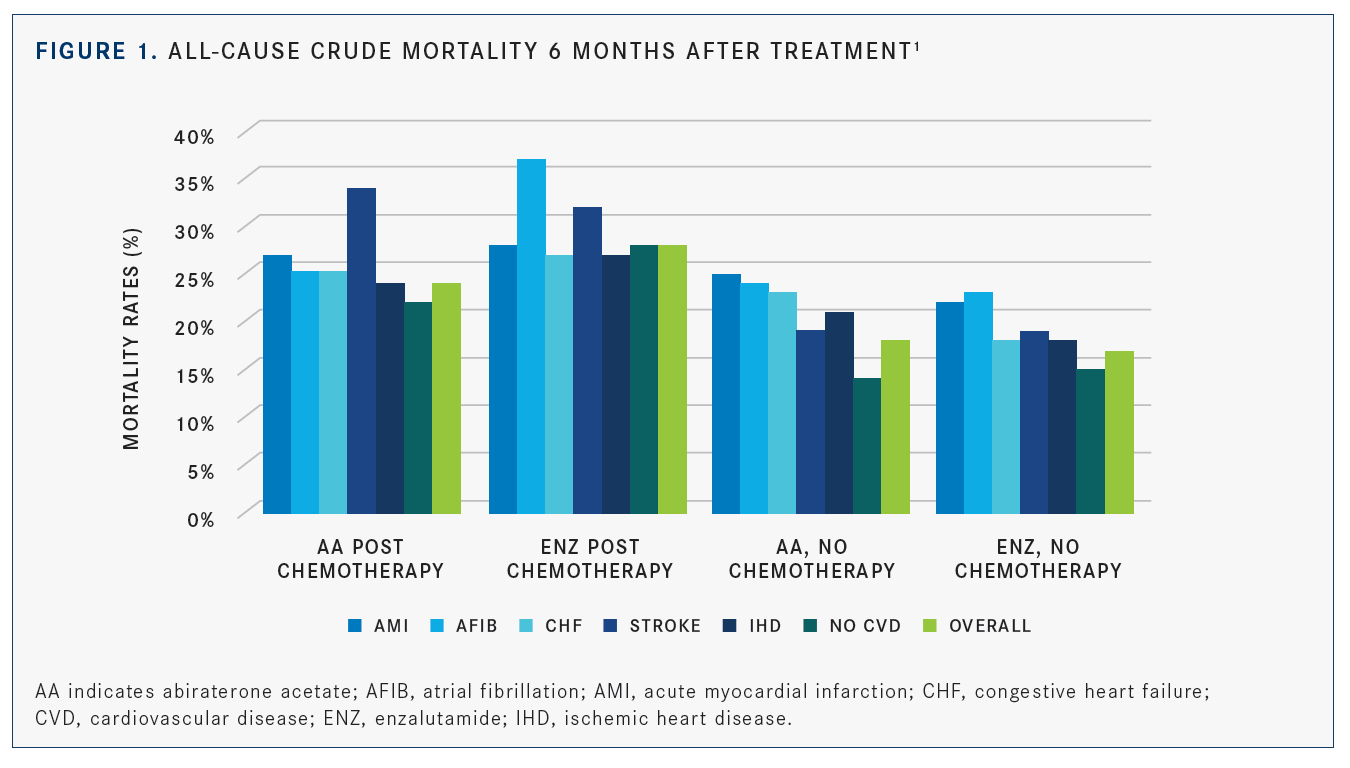
In the population-based study, patients with pre-existing significant cardiovascular condi­tions treated with abiraterone or enzalutamide after docetaxel showed a higher 6-month mor­tality rate compared with pivotal clinical trials results, such as those recorded in COU-AA-301 (NCT00638690) and AFFIRM (NCT00974311). When patients were not given docetaxel before being treated with abiraterone or enzalutamide, they had a higher 6-month crude mortality rate if they also had preexisting CVDs versus patients without them. The mortality rates post chemotherapy and without chemotherapy can be seen inFIGURE 1.1
Whether or not docetaxel was used, ≥3 CVD diagnoses were associated with a higher 6-month mortality relative risk compared with patients without CVDs after abiraterone or enzalut­amide (adjusted relative risk 1.43 for patients treated with docetaxel previously and 1.56 for patients without docetaxel). Most of the survival differences between patients with CVD diagno­ses and patients without CVD diagnoses occurred in the first 6 months of initial treatment with abiraterone or enzalutamide.
“The benefits of the androgen signaling inhibi­tors might not be applicable to patients who have a history of significant [CVDs],” Lu-Yao said. “For patients who do not meet the trial eligibility cri­teria, risk assessment of potential CVD adverse effect and shared decision making is essential. Pretreatment rehabilitation might be needed before the patients start the medication.”
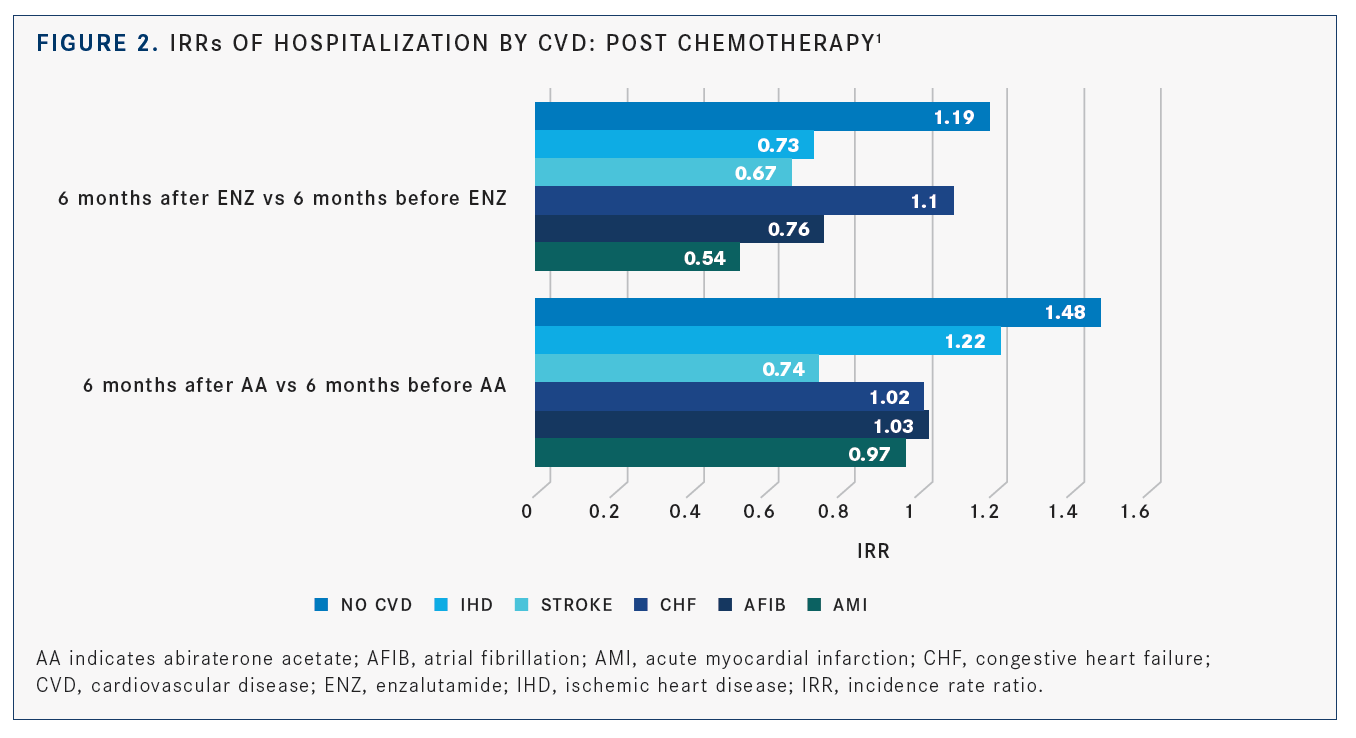
Abiraterone was linked to higher hospitaliza­tion rates irrespective of preexisting CVDs in patients who did not receive docetaxel, based on the adjusted incidence rate ratios (IRRs). It was also associated with a significant increase in posttreatment hospitalization in patients on various classes of medications. Hospitalization rates between abiraterone and enzalutamide showed no significant differences in the post-chemotherapy group, but patients with 1 or 2 CVD diagnoses had a 43% higher risk rate of hospitalization versus patients with no CVDs (adjusted IRR, 1.43; 95% CI, 1.15-1.78), as shown inFIGURE 2.1
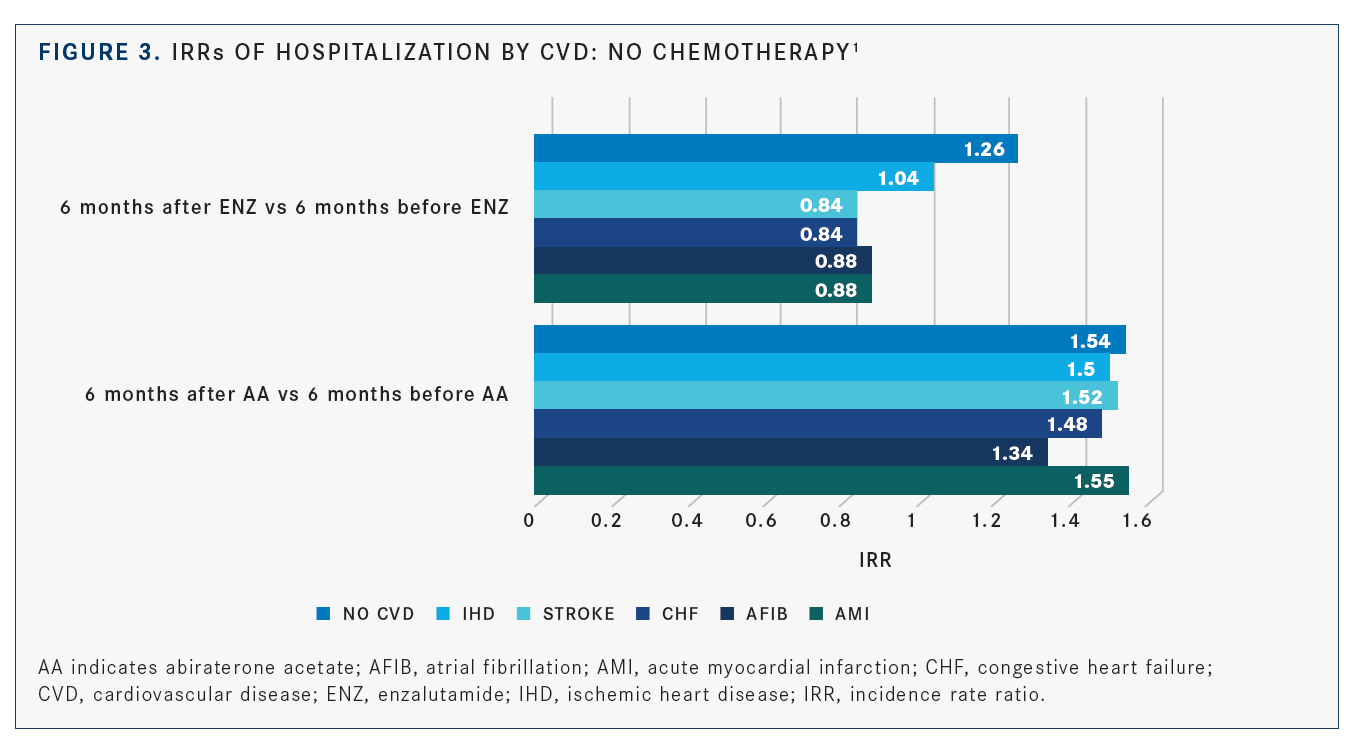
In the no-chemotherapy group, patients with ≥3 CVDs who received enzalutamide had a 41% lower hospitalization rate than those given abi­raterone (adjusted IRR, 0.59; 95% CI, 0.44-0.79). Among the patients in this group, abiraterone was associated with higher rate of hospitaliza­tion post treatment in those with a history of acute myocardial infarction, atrial fibrillation, congestive heart failure, stroke, and ischemic heart disease (FIGURE 3).1
“Many studies have shown that ADT is associated with changes in metabolic profiles and an increased risk of certain CVD condi­tions,” Lu-Yao said. “Because of the concerns about potential adverse effects, most patients with a history of significant CVD conditions are excluded from the pivotal trials of oral androgen-signaling inhibitors. We set out to understand the outcomes of patients who are likely to be excluded from the pivotal trials.”
About 67% of the 3876 eligible patients had at least 1 CVD condition before receiving treatment with abiraterone or enzalutamide. Of the eligible patients, 2845 were treated with abiraterone and 1031 were treated with enzalutamide. The study investigated men who were ≥65 years old and diagnosed with prostate cancer between January 1, 1991, and December 31, 2013. The last follow-up date was December 31, 2015, or the patient’s date of death.
The primary end point was 6-month all-cause mortality from the day patients started abi­raterone or enzalutamide; this was analyzed by modified Poisson regression modeling of relative risk adjusted for confounders and comorbidities. The secondary end point was change in the risk of hospitalization (6 months post-treatment vs 6-month pre-treatment); this was analyzed by negative binomial model to derive adjusted inci­dence rate ratios. These 6-month end points were selected because most adverse CVD events occur within the first 6 months of starting ADT in patients with CVDs.2
“Studies to develop clinical algorithms to identify patients at high risk for cardiovascu­lar adverse events and evaluation of a cardio-rehabilitation intervention will be needed to optimize cancer treatment outcomes,” accord­ing to Lu-Yao.
The postchemotherapy group included patients who received docetaxel before abi­raterone or enzalutamide treatment, and the no-chemotherapy group did not receive docetaxel. The group of patients treated with abiraterone received it alone or before enzalutamide. Likewise, patients treated with enzalutamide received that drug alone or before abiraterone.
Patients who received abiraterone or enzalutamide before docetaxel were excluded due to the small sample size. Also excluded: those who were diagnosed with prostate can­cer at death; did not have Medicare part A, B, or D coverage during the study; or were enrolled in a health maintenance organization.
The preexisting CVDs in the study par­ticipants were acute myocardial infarction (164 patients on abiraterone; 72, enzalut­amide), atrial fibrillation (549, abiraterone; 183, enzalutamide), congestive heart failure (939, abiraterone; 307, enzalutamide), stroke (384, abiraterone; 136, enzalutamide), and ischemic heart disease (1670, abiraterone; 599, enzalutamide). These conditions are not mutually exclusive, which is why the investi­gators divided patients into subsets of those with 0, 1, or 2 ≥3 CVDs.
The investigators’ discussion mentions that this marks the first large-scale, population-based investigation to look at the outcome data after elderly patients with advanced prostate cancer and CVDs received abiraterone or enzalutamide. The data previously were lim­ited because these patients are excluded from clinical trials. These findings suggest taking a multidisciplinary approach when treating patients with advance prostate cancer and pre-existing CVDs, according to the authors.
“Further studies are warranted to under­stand the potential mechanisms and to develop appropriate guidelines to manage this significant subset of men with advanced prostate cancer and comorbidities,” the investigators wrote in their conclusion.
References
- Lu-Yao G, Nikita N, Keith SW, et al. Mortality and hospitalization risk following oral androgen signaling inhibitors among men with advanced prostate cancer by pre-existing cardiovascular comorbidities.Eur Urol. 2020;77(2):158-166. doi: 10.1016/j.eururo.2019.07.031.
- O’Farrell S, Garmo H, Holmberg L, Adolfsson J, Stattin P, Van Hemelrijck M. Risk and timing of cardiovascular disease after androgen-deprivation therapy in men with prostate cancer.J Clin Oncol. 2015;33(11):1243- 1251. doi: 10.1200/JCO.2014.59.1792.

Advances in Subsequent Therapies Shake Up Sequencing of ccRCC Treatment
April 25th 2024With the approval of belzutifan and other newer data for treating patients with recurrent renal cell carcinoma, the state of subsequent therapies is advancing beyond the reuse of frontline options with impacts on duration of response and quality of life.
Read More
Ornstein Advises on Starting Dose and Management of Lenvatinib in RCC
April 21st 2024During a Case-Based Roundtable® event, Moshe Ornstein, MD, MA, provided guidance on dosing and toxicity concerns in a patient treated with lenvatinib plus pembrolizumab for advanced renal cell carcinoma.
Read More