ILCA Data Reignite Interest in AFP as a Biomarker for HCC
Although the use of α-fetoprotein has long been established in hepatocellular carcinoma as a biomarker for screening and diagnosis, its role in treatment selection and prognosis following systemic therapy is a moving target.
Andrew Zhu, MD, PhD

Although the use of α-fetoprotein (AFP) has long been established in hepatocellular carcinoma (HCC) as a biomarker for screening and diagnosis, its role in treatment selection and prognosis following systemic therapy is a moving target.
“AFP is the most widely used marker in HCC and is considered reflective of tumor activity and burden,” Andrew Zhu, MD, PhD, said during a presentation at the International Liver Cancer Association (ILCA) 2020 Virtual Conference. “Early AFP response is also associated with higher efficacy of immune checkpoint inhibitor in advanced HCC.”
An AFP level greater than 400 ng/mL is a prognostic factor for overall survival (OS) in HCC classification systems such as GRETCH (GRoupe d’Etude et de Traitement du Carcinoma Hépatocellulaire), CLIP (Cancer of the Liver Italian Program), and CUPI (Chinese University Prognostic Index), said Zhu. Data from the phase 3 CELESTIAL trial (NCT01908426), reported at ILCA 2019, showed that the AFP level at 8 weeks was the best predictor of OS and progression-free survival (PFS) for cabozantinib (Cabometyx) in patients with previously treated HCC, said Zhu, director of liver cancer research at Massachusetts General Hospital and a professor of medicine at Harvard Medical School, both in Boston.1
At ILCA 2020, key abstracts related to regimens of atezolizumab (Tecentriq) plus bevacizumab (Avastin) in the front line and ramucirumab (Cyramza) in the second line and examined AFP as biomarkers of both prognosis and response in patients with advanced HCC. These results added to previously reported data in this disease setting are explored herein. Considered together, these data may serve to further clarify the role of AFP in therapy monitoring and selection in patients with metastatic tumors.
Overview of AFP Expression in HCC
AFP is a glycoprotein that is produced during fetal stages of development but rapidly declines after birth and typically remains at low levels throughout the remaining life cycle. Hypermethylation of the ZHX2 gene, which silences production of the ZHX2 protein responsible for repressing AFP, occurs in preclinical models of HCC and could explain AFP overexpression in these patients. Additional contributors to increased levels of AFP may include deregulation of pathways impacting liver expression of zinc finger and BTB domain-containing 20 (ZBTB20), another protein that mediates repression of AFP.2
Notwithstanding findings from preclinical research, the mechanism underlying AFP overexpression in patients with HCC remains clouded. Data from patients with HCC remain limited, though a small study of young Peruvian patients with highly atypical HCC presentation showed upregulation of ZHX2 and downregulation of ZBTB20 in tumor specimens. This result contradicts expectations, based on preclinical findings, that both repressors should be downregulated in order to contribute to AFP overexpression.3 Other studies have suggested that effects of cytokine signaling—such as TGFB signaling, which is known to have distinct roles in both inhibition and progression of cancer, depending on disease stage—may also affect AFP expression in patients with HCC.2,4
AFP may also play a role in tumor cell proliferation, but the possible mechanisms behind this are still unclear. Inhibition of apoptotic signal transduction, increased DNA synthesis, and suppression of the antitumor immune response are just some ways in which AFP may promote tumor growth.2
AFP as a Biomarker of Response
A highly vascular disease, HCC growth is dependent on angiogenesis. AFP as a biomarker has been specifically evaluated as a response predictor to antiangiogenic therapies in HCC, and emerging data suggest that there is cross talk between AFP and antiangiogenic vascular endothelial growth factor (VEGF) isoforms.
The use of AFP as a biomarker has already been realized with the use of ramucirumab in patients following frontline treatment with sorafenib (Nexavar). Although ramucirumab failed to show benefit over placebo in patients with advanced HCC following frontline sorafenib in the phase 3 REACH trial (NCT01140347), a prespecified subgroup analysis of patients with elevated baseline serum AFP levels of 400 ng/mL or greater demonstrated significant survival benefit with ramucirumab versus placebo (HR, 0.67; 0.51-0.90; P = .006).2,5
These data led to the initiation of the phase 3 REACH-2 trial (NCT02435433), which showed that ramucirumab met its primary end point of OS improvement versus placebo or best supportive care in patients with AFP levels of 400 ng/mL or greater following sorafenib (HR, 0.71; 95% CI, 0.53-0.95; P = .02). These data ultimately led to the approval of ramucirumab in the indicated patient population.2,6
Interim results of an open-label expansion (OLE) cohort of REACH-2 were presented at ILCA 2020, and these results indicate that ramucirumab may also be effective in patients following prior therapy other than sorafenib.7
Patients treated in the OLE cohort (n = 24) had a median PFS of 5.5 months (95% CI, 1.3-7.5) with second- or third-line ramucirumab following prior therapies such as lenvatinib (Lenvima) (33%), nivolumab (Opdivo, 25%), and PD-1/PD-L1 plus VEGF inhibitor combinations (atezolizumab/bevacizumab, 13%; pembrolizumab [Keytruda] plus lenvatinib, 8%). Partial responses were observed in 4 patients (16.7%), with over half the cohort (54.2%) achieving disease control. Together, these data led investigators to conclude that the efficacy of ramucirumab for these patients was consistent with that observed in patients with prior sorafenib.
AFP as a Prognostic Indicator
Survival following both systemic and local therapy has been closely followed to find potential concordance between AFP response and disease prognosis. One meta-analysis of 29 articles with 4726 patients with HCC showed there was a significant relationship between posttreatment AFP response and OS (HR, 0.41; 95% CI, 0.35-0.47; P < .001) and PFS (HR, 0.46; 95% CI, 0.39-0.54; P < .001). Prior therapies in the analysis included chemotherapy, sorafenib, lenvatinib, radiofrequency ablation, transarterial chemoembolization, and liver transplant.8 At ILCA 2019, Robin Kate (Katie) Kelley, MD, presented data from a subanaly-sis of the CELESTIAL trial showing that cabozantinib improved OS and PFS compared with placebo across a range of baseline AFP levels in the relapsed setting. Consistently, patients with higher baseline AFP levels treated with cabozantinib had shorter OS and PFS than patients in the low-AFP group; however, higher AFP levels were associated with a greater magnitude of benefit with cabozantinib versus placebo therapy.9
“What these data tell us is that baseline AFP level does not predict for any different response on cabozantinib. Rather, …cabozantinib improves PFS and OS independent of the AFP. Stated another way, this suggests that baseline AFP is not a predictive marker for cabozantinib response,” said Kelley. She concluded that AFP response “warrants further evaluation as an early surrogate for response or progression to complement existing radiographic tools while on cabozantinib as well as other systemic ther-apies for advanced HCC, now that there are multiple treatment options available for progression for this population.”
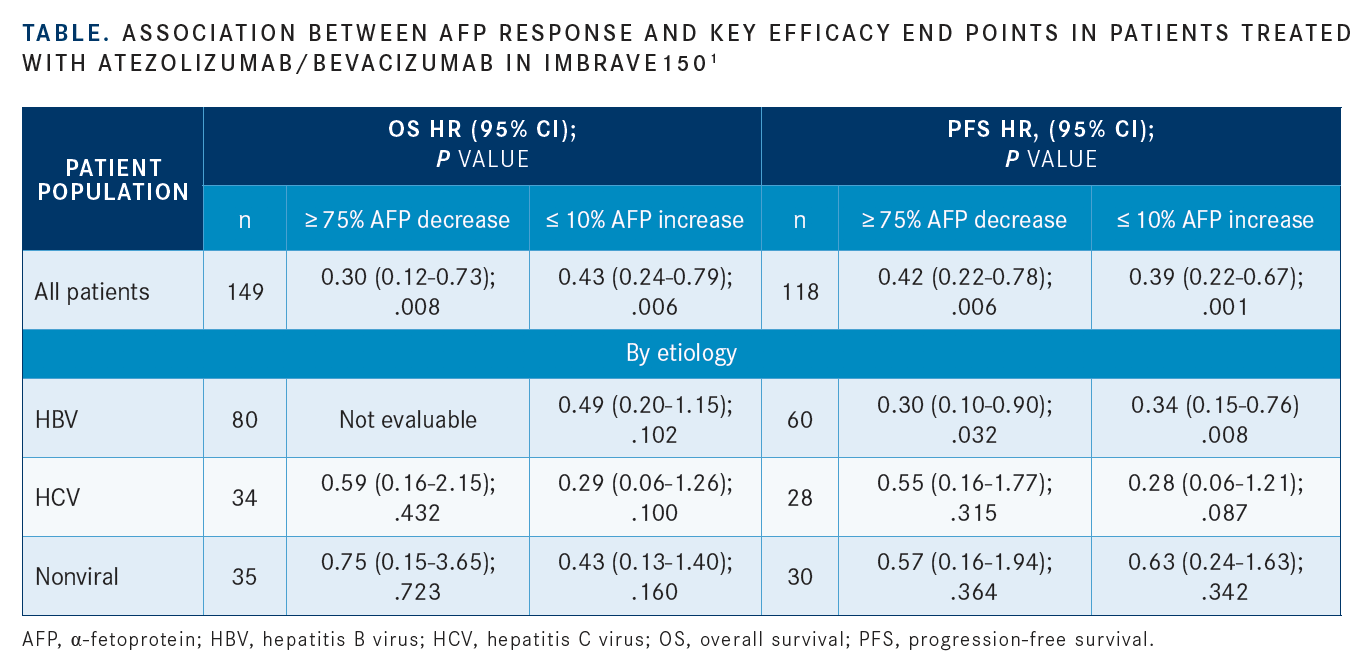
Data recently presented at ILCA 2020 demonstrated that in patients with baseline AFP levels greater than 20 ng/mL who received the atezolizumab/bevacizumab frontline combination in the IMbrave150 trial (NCT03434379), reduction in AFP levels at 6 weeks was significantly associated with OS and PFS improvements versus those without AFP change from baseline (TABLE).1 The phase 3 trial served as the basis of the approval of the PD-L1/VEGF inhibitor combination in patients with treatment-näive HCC. Improvement in both OS and PFS were observed in all patients treated with bevacizumab/atezolizumab versus sorafenib.10
“These AFP cutoffs show potential as surrogate biomarkers of response to atezolizumab/bevacizumab treatment in patients with unresectable HCC,” Zhu said.
Investigators determined optimal AFP cutoffs and time for AFP measurement using data from group A of the phase 1b GO30140 trial (NCT02715531) and further validated them based on data from IMbrave150. They based primary parameters for identifying AFP cutoff levels on differentiating responders from nonresponders (complete and partial responses versus stable and progressive disease) as well as distinguishing between those achieving disease control versus primary progressors. Subsequently, a 75% or greater decrease in AFP was the level chosen to identify responders and a 10% or less increase was chosen to identify those with disease control.1
In the combination therapy arm of the trial, a decrease in AFP levels of 75% or greater significantly improved OS versus those with a decrease of less than 75%, with a hazard ratio of 0.30 (95% CI, 0.12-0.73; P = .008). Patients with hepatitis B virus (HBV) infection and a 75% or greater decrease in AFP levels had significantly better OS and PFS outcomes versus those with less than a 75% decrease.11
Zhu shed some light onto why investigators observed a lack of statistically significant efficacy improvement in the non-HBV subgroups. “I think the sample size is suboptimal for determining the etiology association,” he said. “The HBV-driven HCC is associated in general with high AFP levels. For that reason, perhaps the changes following treatment [are] more reflective of efficacy.”
Similarly, AFP level increases of 10% or less had similar effects on OS (HR, 0.43; 95% CI, 0.24-0.79; P = .006) and PFS (HR, 0.39; 95% CI, 0.22-0.67; P = .001) versus increases of greater than 10%.1
“The threshold for 10% or less increase in AFP levels at 6 weeks may be more clinically relevant for identifying patients who are likely to respond and have prolonged OS from treatment with atezolizumab plus bevacizumab, informing disease management as well as monitoring patients,” Zhu said. “The cut-offs [of AFP levels] were demonstrated to be prognostic of OS and PFS, but further analyses are needed to evaluate these cutoffs for potential as surrogate markers.”
Translating the Data
Regarding prognosis biomarkers, Zhu said imaging studies at critical time points during the disease remain the most effective indicator of patients’ response. AFP levels added to this may serve to further guide clinicians in the treatment of their patients.
“Our data and others’ have increasingly shed additional light on circulating biomarkers, in this case, the use of AFP. In other words, AFP change can serve as a relatively earlier surrogate marker to get a sense of whether the patient is on the right trajectory or not,” Zhu said. “In our study, changes occurring 6 weeks following treatment seem to be the most optimal data that we have. Moving forward, we can at least try to incorporate AFP along with imaging studies to guide the clinical decision.”
References:
1. Zhu AX, Dayyani F, Yen CJ, et al. Alpha-fetoprotein (AFP) kinetics as a potential surrogate biomarker in patients (pts) with hepatocellular carcinoma (HCC) treated with atezolizumab (atezo) + bevacizumab (bev). Presented at: ILCA 2020 Virtual Conference; September 11-13, 2020. Accessed September 15, 2020. https://bit.ly/33wDLsg
2. Galle PR, Foerster F, Kudo M, et al. Biology and significance of alpha-fetoprotein in hepatocellular carcinoma. Liver Int. 2019;39(12):2214-2229. doi:10.1111/liv.14223
3. Marchio A, Bertani S, Rojas Rojas T, et al. A peculiar mutation spectrum emerging from young Peruvian patients with hepatocellular carcinoma. PLoS One. 2014;9(12):e114912. doi:10.1371/journal.pone.0114912
4. Sakata N, Kaneko S, Ikeno S, et al. TGF-β signaling cooperates with AT motif-binding factor-1 for repression of the α-fetoprotein promoter. J Signal Transduct. 2014;2014:970346. doi:10.1155/2014/970346
5. Zhu AX, Park JO, Ryoo BY, et al; REACH Trial Investigators. Ramucirumab versus placebo as second-line treatment in patients with advanced hepatocellular carcinoma following first-line therapy with sorafenib (REACH): a randomised, double-blind, multicentre, phase 3 trial. Lancet Oncol. 2015;16(7):859-870. doi:10.1016/S1470-2045(15)00050-9
6. FDA approves ramucirumab for hepatocellular carcinoma. FDA. May 10, 2019. Accessed September 9, 2020. https://bit.ly/3k5WSjJ
7. Finn RS, De Toni EN, Yau T, et al. Ramucirumab for patients with advanced hepatocellular carcinoma and elevated alpha fetoprotein following a non-sorafenib based systemic therapy: interim results from an expansion cohort of the phase 3 REACH-2 study. Presented at: ILCA 2020 Virtual Conference; September 11-13, 2020. Accessed September 15, 2020. https://bit.ly/33dj0l9
8. He C, Peng W, Liu X, Li C, Li X, Wen TF. Post-treatment alpha-fetoprotein response predicts prognosis of patients with hepatocellular carcinoma: a meta-analysis. Medicine (Baltimore). 2019;98(31):e16557. doi:10.1097/MD.0000000000016557
9. Kelley RK, Bocobo AG, Goyal L, et al. Alpha-fetoprotein (AFP) response and efficacy outcomes in the phase 3 CELESTIAL trial of cabozantinib versus placebo in advanced hepatocellular carcinoma (HCC). Presented at: 13th Annual Conference of the International Liver Cancer Association; September 20-22, 2019; Chicago, IL.
10. FDA approves atezolizumab plus bevacizumab for unresectable hepatocellular carcinoma. FDA. June 1, 2020. Accessed September 14, 2020. https://bit.ly/35BOnZI

Novel Approaches Focus on Limiting Toxicity in Older Patients With ALL
April 22nd 2024The major challenges for clinicians treating older patients with acute lymphoblastic leukemia surround the emergence of resistance to existing therapies and the toxicities associated with current chemotherapies.
Read More
Ornstein Advises on Starting Dose and Management of Lenvatinib in RCC
April 21st 2024During a Case-Based Roundtable® event, Moshe Ornstein, MD, MA, provided guidance on dosing and toxicity concerns in a patient treated with lenvatinib plus pembrolizumab for advanced renal cell carcinoma.
Read More
Rugo Surveys Peers on Treatment After Metastatic Relapse of HR+, HER2– Breast Cancer
April 20th 2024During a Case-Based Roundtable® event, Hope S. Rugo, MD, FASCO, moderated a discussion on treatment options for a patient whose breast cancer recurred several years after adjuvant therapy.
Read More