Bardia Discusses Importance of SERDs in Patients With HR+/HER2– Metastatic Breast Cancer
During a Targeted Oncology™ Case-Based Roundtable™ event, Aditya Bardia, MD, MPH, discussed treatment approaches for a patient with hormone receptor–positive, HER2- negative metastatic breast cancer?
Aditya Bardia, MD, MPH
Director, Breast Cancer Research Program
Massachusetts General Hospital
Associate Professor
Harvard Medical School
Boston, MA

CASE SUMMARY
- A 56-year-old, postmenopausal woman presents with a palpable right breast mass with no clinically abnormal axillary lymph nodes.
- Core biopsy: grade II invasive ductal carcinoma (IDC), estrogen receptor positive (ER+)/progesterone receptor positive (PR+), HER2 immunohistochemistry (IHC) 0, Ki-67 33%
- Lumpectomy and sentinel lymph node (SLN) biopsy: 3.0 cm, grade 2 IDC, 2 SLNs negative for malignant cells
- 21-gene recurrence score is 27
- She receives docetaxel and cyclophosphamide for 4 cycles followed by radiation therapy and completes 5 years of adjuvant aromatase inhibitor (AI).
- 3 years later, she reports right-sided abdominal pain and mild nausea.
- CT scan shows 3 suspicious liver lesions (right lobe, largest 2 cm)
- Liver biopsy shows adenocarcinoma consistent with breast primary ER+/PR+, HER2 IHC 0
- Liver enzymes are normal
- Comprehensive molecular testing from tissue biopsy shows no actionable alterations
- AI plus palbociclib (Ibrance) is initiated.
- Therapy was tolerated well, with grade 2 neutropenia that did not require dose modification of palbociclib.
- 20 months later, follow-up imaging shows increased size of both liver nodules and 2 new lung nodules, largest measuring 0.9 cm
- ECOG performance status is 0; liver enzymes are normal
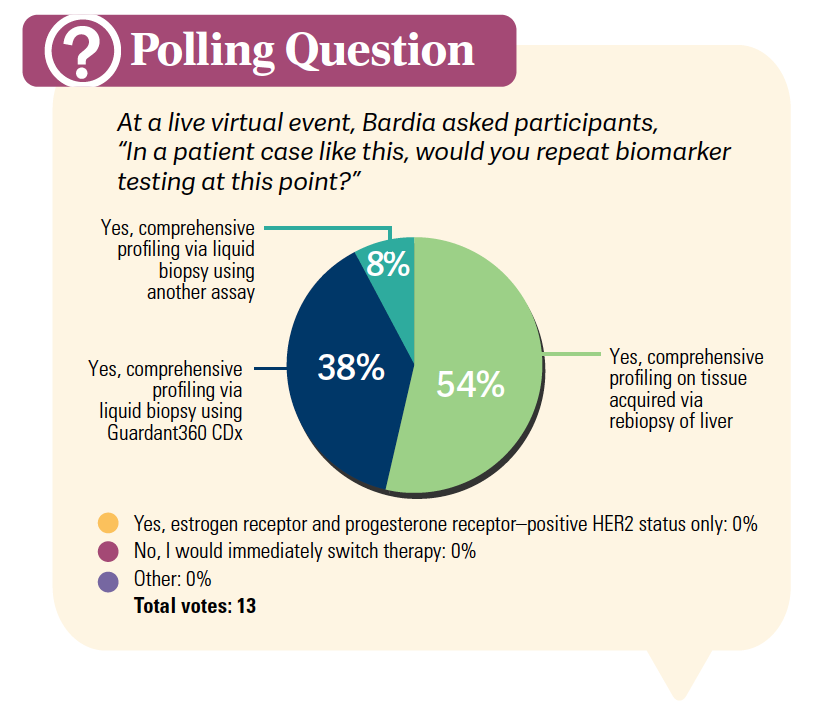
TARGETED ONCOLOGY: If you saw this patient today, what would be your next step?
BARDIA: The standard guidelines say a biopsy should be done at the time of metastatic diagnosis.1 I think everyone would agree that at the first recurrence, we should do a biopsy to confirm that it is indeed cancer and repeat the receptor [testing].
A repeat biopsy could be considered after first-line treatment, or you could consider a liquid biopsy. The idea is to do a comprehensive germline somatic profiling to identify patients who are candidates for additional therapies, as there are a couple targeted FDA-approved therapies available [for these patients].
What therapy options are available for patients with hormone receptor–positive, HER2- negative metastatic breast cancer?
For [patients with] ESR1-mutant tumors, elacestrant [Orserdu] was recently FDA approved.2 The other agents actionable for PIK3CA-mutant tumors that you can consider is fulvestrant [Faslodex] plus alpelisib [Piqray]. This is in the standard-of-care setting. There are clinical trials, as well. Some of these reports usually have the name of the drug that is the potential option in a clinical trial, so the [physician and patient] can consider that.
What are the biomarker testing methods and their associated therapies for treating patients with metastatic breast cancer?
An ESR1 mutation [is] detected by next-generation sequencing, using either [a] blood or tissue [sample]. If you do a repeat biopsy, elacestrant is the recommended treatment. For PIK3CA-mutated tumors, [the recommended treatment] is alpelisib plus fulvestrant.
A couple tumor-agnostic indications include NTRK fusion, for which larotrectinib [Vitrakvi] or entrectinib [Rozlytrek] could be used. This [also] could be [detected] with either tissue or blood. I recently had a patient—the case was published in Therapeutic Advances in Medical Oncology3—with metastatic triple-negative breast cancer who had disease progression on various regimens and was found to have an NTRK fusion, and they did well on larotrectinib. It is rare and is found in [approximately] 1% of breast cancers. [However], if you are seeing circulatory carcinoma, which is rare, you can see an NTRK fusion. Other biomarkers include high microsatellite instability, high tumor mutational burden, and RET fusion.2
CASE UPDATE
Blood-based circulating tumor DNA analysis shows an ESR1 mutation.
What are ESR1 mutations?
ESR1 mutations are mutations in the estrogen or ligand-binding domain, [which is] the site where estrogen binds to the ER. That is the site that acquires a mutation, and because of the mutation, it does not need estrogen to signal, so the switch is constantly on and the tumor becomes estrogen independent.
Drugs [such as] exemestane [Aromasin], letrozole [Femara], and anastrozole [Arimidex] would not work in this setting because the tumor is estrogen independent. [However], it is still ER dependent, so drugs that directly bind to the ER would work in this setting.
Fulvestrant binds to the ER directly, [and so does] tamoxifen [Nolvadex], so in principle, one would expect that these drugs work. The problem is that the doses used in clinic do not degrade or block the ER completely, especially in the presence of an ESR1 mutation [in the tumor].
Do these data support the use of selective ER degraders [SERDs] in patients with ER-positive, HER2-negative, ESR1-mutated metastatic breast cancer?
Based on the preclinical and emerging clinical data, even in the ESR1-mutant setting, you don’t see much benefit with fulvestrant. However, it highlights that this is a setting where if you have a better ER degrader, it would work because the tumor is still ER dependent.
This was first evaluated in the SoFEA [NCT00253422] and EFECT [NCT00065325] trials. These trials looked at fulvestrant vs exemestane in metastatic breast cancer, stratified by ESR1 mutation status. The hypothesis was that exemestane would not work in this setting, but fulvestrant would have some activity. That is what we saw with exemestane in the ESR1-mutant setting, with a median PFS [progression-free survival] of [approximately] 2 months, and 3.9 months for fulvestrant. This was all before CDK4/6 inhibitors. So, there are some differences in the ESR1-mutant setting between fulvestrant and exemestane, but no difference in the wild-type setting.4 [However], even the difference is not that great, which highlights that we need better drugs in this setting.
This is what was then evaluated with elacestrant in the EMERALD [NCT03778931] trial. Other oral SERDs are in development as well. We saw mixed results from these different oral SERDs, but it was partly a study design issue. Many of the trials investigating oral SERDs were not powered to look at the ESR1-mutated subgroup or didn’t have it as a primary end point. So overall, the studies with amcenestrant [SAR439859] or giredestrant [GDC-9545] were negative. But in the ESR1-mutant subgroup, one could see a difference between the oral SERDs and the standard of care, which just highlights that some of the negative results were because of the study design.
How did the EMERALD trial change treatment considerations?
The EMERALD trial had a separate primary end point that looked at the ESR1-mutant subgroup.5 The study was powered to look at that subgroup. This resulted in the approval of elacestrant specifically for this subgroup. The label says [it is for patients with] ER-positive, HER2-negative, ESR1-mutant advanced metastatic breast cancer, [given] after at least 1 prior line of endocrine therapy.
There is no upper limit in terms of prior lines, but you need at least 1 prior line of endocrine-based therapy. The FDA approved Guardant360 CDx as the companion diagnostic,2 but in the clinic, one can use your institutional assay, FoundationOne CDx, or other assays. The EMERALD trial was [conducted] in the second-line setting, and there are a couple unique features about the trial. The first is that it required prior [treatment with a] CDK4/6 inhibitor before enrollment in the trial, which reflects routine clinical practice.
That has an impact on the median PFS. In general, we have seen that across multiple studies in patients who get prior CDK4/6, the response rate or PFS of subsequent therapy—particularly endocrine-based therapy—is lower compared with that in patients who have not received prior CDK4/6 inhibitors.
There is some change in biology, or something happens, that makes it an adverse prognostic group in the second-line plus setting. [However], the duration of the CDK4/6 inhibitor was associated with longer outcomes for patients whose tumors harbored the ESR1 mutation when compared with standard of care [Table6]. The trial had 2 primary end points: PFS in all patients and PFS in patients with the ESR1 mutation [in their tumor]. Randomization was to elacestrant or investigator’s choice of monotherapy.

REFERENCES
1. NCCN. Clinical Practice Guidelines in Oncology. Breast Cancer, version 2.2023. Accessed May 15, 2023. https://tinyurl.com/2khn5xtm
2. FDA approves elacestrant for ER-positive, HER2-negative, ESR1-mutated advanced or metastatic breast cancer. FDA. January 27, 2023. Accessed May 15, 2023. https://tinyurl.com/yfdwjtc5
3. Medford AJ, Oshry L, Boyraz B, et al. TRK inhibitor in a patient with metastatic triple-negative breast cancer and NTRK fusions identified via cell-free DNA analysis. Ther Adv Med Oncol. 2023;15:17588359231152844. doi:10.1177/17588359231152844
4. Turner NC, Swift C, Kilburn L, et al. ESR1 mutations and overall survival on fulvestrant versus exemestane in advanced hormone receptor-positive breast cancer: a combined analysis of the phase III SoFEA and EFECT trials. Clin Cancer Res. 2020;26(19):5172-5177. doi:10.1158/1078-0432.CCR-20-0224
5. Bidard FC, Kaklamani VG, Neven P, et al. Elacestrant (oral selective estrogen receptor degrader) versus standard endocrine therapy for estrogen receptor-positive, human epidermal growth factor receptor 2-negative advanced breast cancer: results from the randomized phase III EMERALD trial. J Clin Oncol. 2022;40(28):3246-3256. doi:10.1200/JCO.22.00338
6.Bardia A, Bidard FC, Neven P, et al. EMERALD phase 3 trial of elacestrant versus standard of care endocrine therapy in patients with ER+/HER2- metastatic breast cancer: updated results by duration of prior CDK4/6i in metastatic setting. Presented at: San Antonio Breast Cancer Symposium; December 6-10, 2022; San Antonio, TX. Abstract GS3-01.
