Bryce Reviews PARP Inhibitor Combination Trials in Prostate Cancer
During a Targeted Oncology™ Community Case Forum event in partnership with The Arizona Clinical Oncology Society, Alan H. Bryce, MD, reviewed data from clinical trials utilizing PARP inhibitors in patients with prostate cancer.
Alan H. Bryce, MD
Professor of Medicine
Chair of the Division of Hematology and Medical Oncology
Department of Internal Medicine
Mayo Clinic
Phoenix, AZ

CASE SUMMARY
- Patient is a 65-year-old man
- Had hip replacement at age 58
- Diagnosed with localized prostate cancer
- At initial diagnosis:
- Serum prostate-specific antigen (PSA): 15.7 ng/mL
- Prostate biopsy: Gleason 7 (4 + 3) prostate adenocarcinoma
- Baseline staging: T3bN1M0 with right seminal vesicle and pelvic lymph node involvement
- Underwent radical prostatectomy with pelvic lymph node dissection
- PSA nadir of 0.4 ng/mL post surgery
- Gleason 7 (4 + 3) confirmed
- Positive surgical margins
- American Joint Committee on Cancer stage IVA
- Two months later:
- Serum PSA, 38 ng/mL; alkaline phosphatase (ALP), 289 IU/L
- PSMA (prostate-specific membrane antigen) PET/CT:
- Metastatic retroperitoneal lymph nodes (nodes outside resection field)
- Abdominal nodes
- Bone metastases in ribs and thoracic spine
- Genetic testing: BRCA2 mutation–positive
- Abiraterone plus prednisone initiated
- Improved pain, PSA, and ALP
- Eight months later:
- Patient reported bone pain
- Laboratory testing revealed rising PSA and ALP (serum PSA, 350 ng/mL; ALP, 2500 IU/L)
- Imaging showed radiologic progression
- ECOG performance status: 1
- PSMA PET/CT: increased uptake in retroperitoneal and abdominal lymph nodes
- Treated with docetaxel chemotherapy for 4 cycles and bone protecting agent
- Serum PSA, 38 ng/mL; ALP, 289 IU/L
- Reporting bone pain increased in back, now reporting pain in hip area
- Bone scan and CT scan:
- Enlargement of retroperitoneal and abdominal lymph nodes
- Additional bone metastases noted in pelvic region
TARGETED ONCOLOGY: What do the NCCN (National Comprehensive Cancer Network) guidelines recommend for subsequent systemic treatment of metastatic castration-resistant prostate cancer (mCRPC)?
BRYCE: For patients with CRPC who have a pathogenic variant and a DNA damage response [DDR] gene, we have 2 drugs that have been approved.1 We have olaparib [Lynparza], which has a broader label indication across multiple DDR genes both pre- and post-chemotherapy.2 We also have rucaparib [Rubraca], which has a narrower indication, just for BRCA1/2-[mutated] cancer in the postdocetaxel [Taxotere] space.3
This patient could have received olaparib prior to his docetaxel. Post docetaxel, he can receive either drug, because he has a BRCA germline mutation. But with olaparib, you have more flexibility pre- and post-dosing, and a longer list of genes, whereas rucaparib is more restricted.
Can you discuss the evidence for olaparib in mCRPC with progression on androgen receptor pathway inhibitors?
PROfound [NCT02987543] was a study looking at olaparib.4 It has a longer list of qualifying genes. BRCA1/2, ATM, and PALB2 were significant, with some responses there, but [there is] a longer list. All the patients on this study had to have received at least 1 prior androgen receptor pathway inhibitor, abiraterone [Zytiga] or enzalutamide [Xtandi] in this case, because of the years in which the study was accrued. They could have received docetaxel, but they didn’t have to, so there was a mix of pre- and post-docetaxel patients. They were randomly assigned to receive olaparib or the other androgen receptor pathway inhibitor that they hadn’t received before, either enzalutamide or abiraterone.
The patients were put into 2 cohorts, [those with mutations in] BRCA1/2 or ATM vs all the other alterations. The primary end point is radiographic progression-free survival [rPFS], and the secondary end points [included] overall survival [OS], objective response rate, etc.
The biomarkers were looked at retrospectively. [Mutated] BRCA2 is more common in prostate cancer than BRCA1, [at a ratio of] 5:1 or 10:1. This is very different from the population of patients with ovarian cancer [or] breast cancer, this distribution of the qualifying aberrations.
ATM ends up being the most common non-BRCA aberration, and then the rest end up being fairly uncommon. With ATM, one of the problems we have in prostate cancer is, if you’re detecting it by cell-free DNA, probably 50% of those are actually CHIP [clonal hematopoiesis of indeterminate potential] and not prostate cancer, and that’s a bit of a problem in terms of understanding if you have a real ATM mutation or not.
When you look at the subgroup analysis, it is consistent with every PARP inhibitor study we have in prostate cancer; the best responses are in the BRCA2 group. The BRCA2 group is different than all others. That is not drug-specific or not trial-specific at all.
BRCA1-aberrant disease response [being worse] than BRCA2 is a consistent finding.4 For ATM-positive disease, the HR is 1.04 [95% CI, 0.61-1.87]. Nevertheless, it’s in cohort A in this study. In the rest of these [mutations], the strongest signal we see across multiple studies is usually from PALB2.
What were the data for olaparib with abiraterone as first-line treatment for mCRPC?
PROpel [NCT03732820] was a very important study, because it was the first to report out on a large-scale randomized trial combining olaparib with abiraterone as the [first-line] treatment for mCRPC.5 Importantly, this was in all comers, not just biomarker-selected patients, so this includes [patients with BRCA mutations as well as those with wild-type BRCA].
Patients could have received docetaxel for their first-line mHSPC [metastatic hormone-sensitive prostate cancer], but they could not have received it in the castration-resistant setting, and they could not have been on prior NHAs [novel hormonal agents] leading into the study. The primary end point here is rPFS, and a secondary end point is OS.
The percentage of [patients with HRRm [homologous recombination repair mutations] is a little bit high, close to 30%. We’d expect 20% to 25%, so there is a slight amount of referral bias.6 Only 2.3% of patients were undefined, and this would be because of testing failure. Everybody had samples collected, but testing is not always going to work. Nothing stands out to say that this population is particularly aberrant or particularly low or high risk.
The primary end point of rPFS was positive, favoring the combination arm of olaparib and abiraterone. The HR was very nice at 0.66 [95% CI, 0.54-0.81; P < .0001]. Median OS on the combination arm was 24.8 months vs 16.6 months [with abiraterone plus placebo], an 8-month improvement. This is clinically meaningful as well. The [patients with BRCA mutations] have dramatically more benefit than all others. This is consistent and…is what you’d expect. They do better, but nevertheless, and importantly, you still see benefit in the non-HRR patients [HR, 0.76; 95% CI, 0.60-0.97] and in the non-BRCA patients [HR, 0.76; 95% CI, 0.61-0.94].
The OS has an HR that is not quite as good, but still good, 0.81 [95% CI, 0.67-1.00], with a P value of .377, and median OS of 42.1 months with olaparib vs 34.7 months without olaparib. Of course, one of the things we always take away here…in first-line mCRPC, once we get to this point, we’re talking about 3 years, more or less, of overall survival. Maybe 5 years overall nowadays, but 3 years [with] mCRPC, 5 years [with] mHSPC.
Then we get the breakdown, and again, no question, the HRRm population gets more benefit than the all-comers population, so you see that top line here or there. For PFS and for OS, the questions start to come up, the BRCA is dramatically positive. This is the HR for PFS, 0.29 [95% CI, 0.14-0.56] for BRCA [patients]; for the non-BRCA [patients], now you’re talking ATM, etc, that HR is only 0.91 [95% CI, 0.73-1.13].
[Looking at] OS by the different populations, the curves, obviously, are widely separated in the HRRm population [HR, 0.66; 95% CI, 0.45-0.95]. The curves aren’t that separated in the non-HRRm population. The HR there is 0.89 [95% CI, 0.70-1.14].
These [data] are not at full maturity, and there is the potential for crossover later. There is the potential for subsequent treatments…[and] there is a potential for these curves to separate with more maturity, but for the OS across subgroups, the [patients with BRCA mutations] get the dominant benefit [Table6].
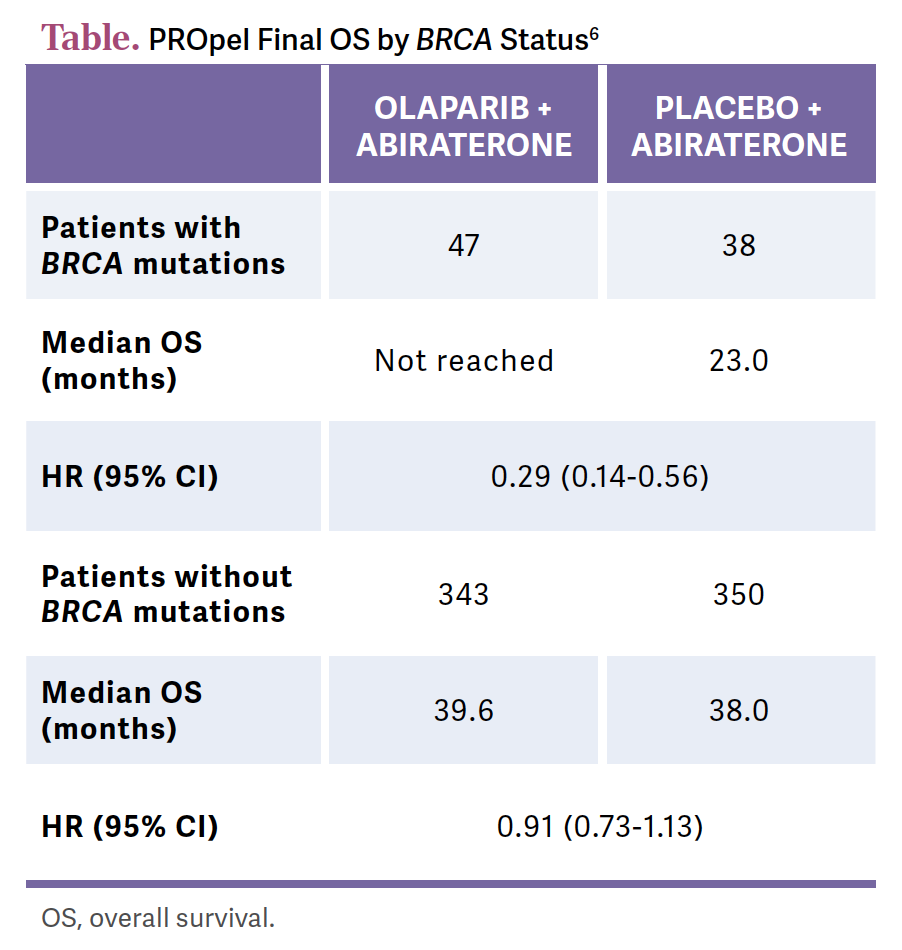
The [confidence intervals for patients without BRCA mutations] don’t cross 1. We’ve had this with other therapies in other settings. If there’s a PFS benefit, and you don’t see a strong OS benefit, how do we feel about that result? Our gynecologic oncology colleagues can certainly relate to this conversation.
What safety outcomes were seen with olaparib plus abiraterone?
There are additive toxicities…what you’d expect to see from the 2 drugs separately when you put them together.6 In terms of anemia, in terms of fatigue, you have a combination of PARP plus abiraterone toxicities. There’s no synergistic toxicity with this combination.
Deaths due to adverse events [AEs] were [rare]. Discontinuation was more common in the combination arm because we’re holding PARP inhibitors for cytopenias and anemia.
There were 2 cases of MDS/AML [myelodysplastic syndrome/acute myelocytic leukemia] in the combination arm. In patients with prostate cancer, we don’t have the same amount of follow-up as we have in ovarian cancer. We actually pay attention to the ovarian cancer data for this issue. As we move PARP inhibitors earlier, giving patients longer exposure, are we worried about this?
These patients, compared with [those in] the breast and ovarian studies, are somewhat older. A lot of them have had pelvic radiation. The bone marrow reserve becomes a real management issue later in the disease course. Patients with prostate cancer [are often] limited by the fact that their marrows are ablated and their blood cell counts are not there, and treatment options are limited by the fact that hemoglobin won’t go above 9.5 g/dL.
How did rucaparib do in patients with chemotherapy-naïve mCRPC?
I presented the TRITON3 study [NCT02975934] at the American Society of Clinical Oncology Genitourinary Cancers Symposium [ASCO GU], so I know these data very well.7 This is the randomized phase 3 trial of rucaparib in the chemotherapy-naïve setting.8 The initial approval is based on TRITON2 [NCT02952534], and that is in the postdocetaxel setting.9 This is the confirmatory study, predocetaxel, in patients only with BRCA or ATM alterations, and in patients who have not received taxane chemotherapy, except in the hormone-sensitive setting.7
Patients were randomly assigned 2:1 between the control arm and rucaparib, and significantly, in this study, the control arm included docetaxel, and it was physician’s choice.8 We had to determine this up front, but…more patients got docetaxel than the second-generation androgen receptor pathway inhibitor. Crossover was built into the study, so 75% of patients crossed over to receive rucaparib after being on the physician’s choice arm.
The primary end point was rPFS in the BRCA subgroup. This was prespecified. The intent was to focus specifically on this group. There was a clearly positive response here. The HR was 0.61 [95% CI, 0.47-0.80], with a P value of .0003, and the median rPFS in the BRCA subgroup was 11.2 months with rucaparib vs 6.4 months [in the control group].7 The intent-to-treat population is all patients, [those with altered] BRCA plus ATM, and we don’t see response in ATM, so the numbers come down with the ATM [subgroup]. The ATM subgroup shows basically no benefit, [and so] I don’t use PARP inhibitors in ATM-mutant disease.
For the interim OS, the data are not yet mature. [We looked at OS] by the chemotherapy treatment or by the choice of control arm. In the chemotherapy-treated group, the median OS was 18.9 months vs 24.3 months [HR, 0.75; 95% CI, 0.51-1.11] with rucaparib. In the second-generation androgen receptor pathway inhibitor group, it was 22.1 months vs 24.3 months with rucaparib [HR, 0.81; 95% CI, 0.52-1.27]. The numbers get quite small when we’re talking about the breakdown of the physician’s choice of therapy.
There were no surprises [in terms of] safety. Importantly, the prostate studies were the first time the PARP inhibitors had been used in men, so there was a question whether there would be a gender difference in toxicity. There is not. It’s equivalent—[which was] important to prove. [The most common AEs are] anemia, GI [gastrointestinal] toxicity, myelosuppression, and fatigue. In the control arm, there are differences [in safety] for docetaxel vs the oral agents.
What are the data for niraparib (Zejula) and abiraterone as first-line therapy?
MAGNITUDE [NCT03748641] was another combination study with different results than we see in PROpel.10 This is niraparib and abiraterone in mCRPC. In this study, they had separate cohorts for the [patients with HRR mutations] vs [those with] wild-type with a futility analysis plan for each arm, placebo-controlled randomization, with rPFS as the primary end point.
BRCA2 was the most common…aberration. There were slight imbalances in terms of the visceral metastases between the groups, etc. About 20% of patients had taxanes in the hormone-sensitive setting.
In this study, [since] the HRR wild-type patients were in a separate cohort, they did a futility analysis showing no benefit. The enrollment to that cohort was cut off after this interim analysis, and they didn’t move on. So 233 patients enrolled and then discontinued. However, in the [patients with BRCA1/2 mutations], there was a strongly positive result [HR, 0.53; 95% CI, 0.36-0.79; P = .0014] favoring combination therapy over abiraterone alone.
At the second interim analysis, the benefit is maintained primarily in the BRCA subgroup. The BRCA subgroup does better than the all-HRR population. BRCA is always where we see the most benefit concentrated.
In [terms of] treatment-emergent AEs, there are some slight differences between the PARP inhibitors. There are slight differences in the rate of myelosuppression, and slight differences in some drug-drug interactions. I would not treat the PARP inhibitors as interchangeable. The androgen receptor pathway inhibitors are also not interchangeable. I would assess each trial separately.
Can you discuss talazoparib (Talzenna) in combination with enzalutamide for mCRPC?
TALAPRO-2 [NCT03395197] was the third combination study, and this is why I say to treat each combination separately.11 There was a lot of conversation…that this is going to be the tie-breaking study. I don’t think that’s true. Talazoparib is a very different PARP inhibitor than the others. It was combined with enzalutamide in a randomized study in the predocetaxel setting, and they included both [patients with HRR mutations and those with HRR wild type]. [There was] 1:1 randomization, and the primary end point [was] rPFS, so this was the FDA-directed design for these studies, focus on rPFS as a primary end point [and] OS as the secondary end point.
The initial result, not the final result, was presented…at ASCO GU.12 In this study, 21% to 23% received docetaxel in the hormone-sensitive setting. Over 20% of patients were HRR deficient. That is keeping with the overall prevalence.
Again, rPFS was positive, we’re getting used to the HR again falling in this range, 0.63 [95% CI, 0.51-0.78; P < .001], favoring the combination arm over the enzalutamide monotherapy arm. When we break it down by HRR deficient vs nondeficient or unknown, there is a more profound effect in the HRR-deficient population, with an HR of 0.46 [95% CI, 0.30-0.70; P < .001], vs 0.70 [95% CI, 0.54-0.89; P = .004] in the unknown or nondeficient population.
Unknown HRR status becomes somewhat controversial. We’re defining HRR differently in different tumors. Is there HRR deficiency, outside of how we’re defining it in prostate cancer, driving this? I think it’s a question we have to answer.
Treatment-emergent [AEs] again [included] myelosuppression and cytopenias. We’re all familiar with the enzalutamide toxicities. About 8.3% of patients needed dose reductions with the talazoparib. There is a drug-drug interaction with talazoparib and enzalutamide, which is why you see a dose reduction of the talazoparib over what you might be accustomed to seeing from other studies.
REFERENCES
1. NCCN. Clinical Practice Guidelines in Oncology. Prostate cancer, version 1.2023. Accessed April 21, 2023. https://tinyurl.com/kxx4ajxx
2. Lynparza. Prescribing information. AstraZeneca; 2019. Accessed August 7, 2023.https://tinyurl.com/4pm9trfr
3. Rubraca. Prescribing information. Clovis; 2022. Accessed August 7, 2023. https://tinyurl.com/2p8bhvv3
4. De Bono J, Mateo J, Fizazi K, et al. Olaparib for metastatic castration-resistant prostate cancer. N Engl J Med. 2020;382(22):2091-2102. doi:10.1056/NEJMoa1911440
5. Clarke NW, Armstrong AJ, Thiery-Vuillemin A, et al. Abiraterone and olaparib for metastatic castration-resistant prostate cancer. NEJM Evidence. 2022;1(9):EVIDoa2200043. doi:10.1056/EVIDoa2200043
6. Clarke NW, Armstrong AJ, Thiery-Vuillemin A, et al. Final overall survival (OS) in PROpel: Abiraterone (abi) and olaparib (ola) versus abiraterone and placebo (pbo) as first-line (1L) therapy for metastatic castration-resistant prostate cancer (mCRPC). J Clin Oncol. 2023;41(suppl 6):LBA16. doi:10.1200/JCO.2023.41.6_suppl.LBA16
7. Bryce AH, Piulats JM, Reaume MN, et al. Rucaparib for metastatic castration-resistant prostate cancer (mCRPC): TRITON3 interim overall survival and efficacy of rucaparib vs docetaxel or second-generation androgen pathway inhibitor therapy. J Clin Oncol. 2023;41(suppl 6):18. doi:10.1200/JCO.2023.41.6_suppl.18
8. Fizazi K, Piulats JM, Reaume MN, et al. Rucaparib or physician’s choice in metastatic prostate cancer. N Engl J Med. 2023;388(8):719-732. doi:10.1056/NEJMoa2214676
9. Abida W, Patnaik A, Campbell D, et al. Rucaparib in men with metastatic castration-resistant prostate cancer harboring a BRCA1 or BRCA2 gene alteration. J Clin Oncol. 2020;38(32):3763-3772. doi:10.1200/JCO.20.01035
10. Chi KN, Rathkopf DE, Smith MR, et al. Phase 3 MAGNITUDE study: first results of niraparib (NIRA) with abiraterone acetate and prednisone (AAP) as first-line therapy in patients (pts) with metastatic castration-resistant prostate cancer (mCRPC) with and without homologous recombination repair (HRR) gene alterations. J Clin Oncol. 2022;40(suppl 6):12. doi:10.1200/JCO.2022.40.6_suppl.012
11. Agarwal N, Azad A, Shore ND, et al. Talazoparib plus enzalutamide in metastatic castration-resistant prostate cancer: TALAPRO-2 phase III study design. Future Oncol. 2022;18(4):425-436. doi:10.2217/fon-2021-0811
12. Agarwal N, Azad A, Carles J, et al. TALAPRO-2: Phase 3 study of talazoparib (TALA) + enzalutamide (ENZA) versus placebo (PBO) + ENZA as first-line (1L) treatment in patients (pts) with metastatic castration-resistant prostate cancer (mCRPC). J Clin Oncol. 2023;41(suppl 6):LBA17. doi:10.1200/JCO.2023.41.6_suppl.LBA17

Bispecific Antibodies and ADCs Deliver a Futuristic Horizon Across Lung Cancer Settings
October 23rd 2024Recent advancements in protein engineering, especially antibody-drug conjugates, show promise in lung cancer treatment, with ivonescimab outperforming pembrolizumab in PD-L1-positive advanced non-small cell lung cancer.
Read More