Clinical Trials Are Underway on Novel Approaches to CML-Resistant BCR-ABL1 Inhibitors
Agents with novel mechanisms of action distinct from available ATP-competitive tyrosine kinase inhibitors have been tested in patients with previously treated, Philadelphia chromosome–positive chronic myeloid leukemia and offer promise to facilitate deeper remissions.
Jorge E. Cortes, MD
Agents with novel mechanisms of action distinct from available ATP-competitive tyrosine kinase inhibitors (TKIs) have been tested in patients with previously treated, Philadelphia chromosome–positive (Ph+) chronic myeloid leukemia (CML) and offer promise to facilitate deeper remissions.
Jorge E. Cortes, MD, director of the Georgia Cancer Center at Augusta University, said key considerations for treating this patient population are achieving sufficiently deep responses to consider treatment discontinuation and improving quality of life; however, challenges to realizing these goals remain.
“[Existing agents for treating CML] work very well. So, showing improvement becomes a little harder,” Cortes said in an interview with Targeted Therapies in Oncology.
“I think there is still room for improvement. We still don’t get enough patients to the deepest molecular responses so they can consider treatment discontinuation.”
Almost all cases of CML are driven by the presence of the Philadelphia chromosome, the result of a translocation event producing the BCR-ABL1 fusion gene. The resulting hybrid protein possesses dysregulated tyrosine kinase activity.1 Although patients with CML generally do well on current treatments and can have a near-normal life expectancy, there are aspects of CML therapy that could be improved.
Developing New Strategies to Address Unmet Needs in Standard Treatment Sequences
Newly diagnosed chronic-phase (CP) CML is effectively treated with currently available TKIs, including the first-generation BCR-ABL1 inhibitor imatinib (Gleevec) and second-generation agents dasatinib (Sprycel), nilotinib (Tasigna), and bosutinib (Bosulif). Mutations in the BCR-ABL1 kinase domain, such as T315I, frequently confer resistance to TKIs. The third-generation ponatinib (Iclusig) is the only FDA-approved TKI active against CML with the T315I mutation, which renders the disease completely resistant to other BCR-ABL1–targeting agents.1 However, ponatinib is associated with potentially life-threatening adverse effects (AEs).2
As there are few options for CML-CP resistant to multiple TKIs,1 there is an unmet medical need for new therapies for patients who have TKI-resistant CML or who cannot tolerate the toxicity associated with current agents.3
Currently available TKIs for CML are orthosteric inhibitors, all of which directly target the ATP-binding site of the BCR-ABL1 kinase.3,4
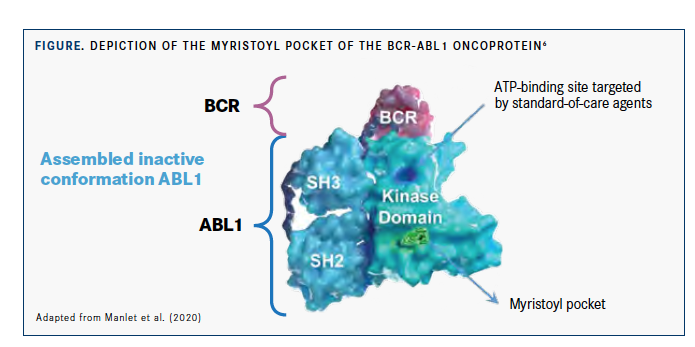
The wild-type ABL1 kinase is myristoylated at its N-terminal end. This myristoyl group can bind to a myristoyl pocket in the kinase domain of the molecule, changing ABL1’s conformation and acting as an allosteric auto-inhibitor to keep its kinase activity in check. During the translocation event that produces BCR-ABL1, the myristoylated ABL1 region is lost and the kinase becomes constitutively active. Therefore, the myristoyl pocket, which is located in a different region of the kinase domain from the ATP-binding site, provides a new therapeutic target (FIGURE).3-6
Recent data from a phase 3 trial demonstrated that the novel investigational agent asciminib (ABL001), which targets the ABL1 myristoyl pocket, was superior to bosutinib in producing a major molecular response (MMR) in patients with Ph+ CML.
As an allosteric inhibitor that Specifically Targets ABL1 Myristoyl Pocket (STAMP), the novel agent asciminib is active against BCR-ABL1 with resistance mutations in the ATP-binding site, including T315I, and is expected to have lower off-target activity, resulting in a better safety profile compared with ATP-competitive TKIs.3,7
Preclinical Development of STAMP Inhibition for Ph+ CML
In preclinical studies, asciminib inhibited the growth of primary cells from patients with CML as well as human Ph+ leukemia cell lines but did not inhibit cells from healthy donors. Asciminib also inhibited the growth of some, but not all, cell lines with point mutations in BCR-ABL1 that confer resistance to approved TKIs. Combining asciminib with either nilotinib or ponatinib largely overcame resistance to single-agent asciminib.8
The preclinical activity of asciminib formed the basis for testing it in clinical trials. The allosteric inhibitors GNF-2 and GNF-5 have structures similar to that of asciminib, and, like asciminib, they both bind to the myristoyl pocket of BCR-ABL1. Although GNF-2 and GNF-5 have preclinical activity, they have not been tested in clinical trials in CML.9
Clinical Development of Asciminib for Ph+ CML
In an ongoing, phase 1, study (NCT02081378), asciminib was administered (as monotherapy or in combination with nilotinib, imatinib, or dasatinib) to patients with heavily pretreated CML that had relapsed or was refractory to prior TKIs. In a report of findings for the asciminib monotherapy arm, most of the 150 enrolled patients (n = 141) had CML-CP.10 Median follow-up was 59 weeks, and the maximum tolerated dose of asciminib was not reached.10
Among the 113 patients with CML-CP without the T315I mutation, a complete hematologic response (CHR) was achieved in 34 of 37 (92%) who did not have CHR at baseline, and a complete cytogenetic response (CCR) was seen in 31 of 57 (54%) patients without CCR at baseline. MMR (defined as BCR-ABL1IS ≤0.1%, ie, presence of the BCR-ABL1 fusion in ≤0.1% of cells in a blood sample) was achieved or maintained by 12 months in 44 of 91 (48%) evaluable patients without T315I and in 5 of 18 patients (28%) with T315I at baseline.10
Dose-limiting toxicities (DLTs) of asciminib monotherapy included pancreatitis and asymptomatic elevated lipase levels. Common AEs included fatigue, headache, arthralgia, hypertension, and thrombocytopenia.10 Asciminib treatment is also being assessed in a larger cohort of patients with the T315I mutation in this study.3,11'
In the same trial, asciminib monotherapy was examined in a cohort of 48 patients with BCR-ABL1IS ≤1% at baseline, a population that is especially likely to experience intolerance of traditional TKIs. At a median duration of treatment of just over 3 years, 87.5% of patients remained on treatment, and 75% had achieved MMR or better. The most common grade 3/4 AEs were increased lipase and hypertension.12
The asciminib plus imatinib arm of this study enrolled 25 patients with CML-CP. MMR was observed by 48 weeks in 8 of 19 (42%) evaluable patients who did not have MMR at baseline.13
Grade 3/4 treatment-related AEs (TRAEs) occurred in 44% of patients. DLTs included decreased neutrophil count, abdominal pain, nausea, pancreatitis, and increased lipase. The most common any-grade AEs were nausea, increased lipase, abdominal pain, peripheral edema, and vomiting.13
The asciminib plus nilotinib and asciminib plus dasatinib arms of this study each enrolled 17 patients; all but 1 patient in each arm had CML-CP. The choice of agent to combine with asciminib was based on prior intolerance. In the nilotinib group, there was 1 DLT of maculopapular rash during the first cycle. Additionally, 53% of patients had grade 3/4 TRAEs, with the most common any-grade AEs being myalgia, increased lipase, increased amylase, fatigue, and pruritus. In the dasatinib group, there was 1 DLT of increased lipase during cycle 1; 29% of patients had grade 3/4 TRAEs, with the most common any-grade AEs being increased lipase, diarrhea, headache, and nausea. Approximately one-third of patients in each arm without MMR at baseline experienced MMR by 48 weeks.14
The ASC4MORE trial is an ongoing phase 2, open-label, randomized efficacy and safety study (NCT03578367) recruiting an estimated 80 patients with CML-CP who have not experienced a deep molecular response (BCR-ABL1IS ≤0.01%) on frontline imatinib. Patients are randomized to either continue imatinib monotherapy, add asciminib (40 mg or 60 mg) to the imatinib regimen, or switch to nilotinib.3
The ongoing phase 3 ASCEMBL study (NCT03106779) is evaluating asciminib monotherapy versus bosutinib in patients with CML-CP who have previously been treated with at least 2 ATP–binding-site TKIs.3 Patients are randomly assigned to asciminib 40 mg twice daily (n = 148) or bosutinib 500 mg once daily (n = 74). Patients who experience treatment failure on bosutinib are allowed to cross over to asciminib. The primary end point is MMR at 24 weeks, with a key secondary end point of MMR at 96 weeks. Other secondary objectives include efficacy and safety.3
At primary analysis, the ASCEMBL trial was reported to have met its primary end point. Asciminib was associated with statistically significant superiority in terms of the MMR rate at 24 weeks compared with bosutinib, although investigators have not reported any specifics. Data will be presented at an upcoming medical meeting. Novartis, the manufacturer of the agent, reported that the FDA has granted asciminib a fast track designation.5
Other Potential Agents for Ph+ CML
Agents with mechanisms of action differing from TKIs targeting the ATP-binding site of BCR-ABL1 have been tested in Ph+ CML, particularly in patients who have the T315I mutation or have experienced treatment failure with TKIs approved for CML, and the results have been mixed. The farnesyl transferase inhibitors lonafarnib (SCH 66336) and tipifarnib (Zarnestra; R115777) were tested as monotherapies or in combination with imatinib in small, early-phase studies (NCT00047502; NCT00038597; NCT00040105), after which their development in CML was apparently halted.15,16
Other agents that have been tested in TKI-resistant CML include pan-aurora kinase (serine/threonine kinase) inhibitors, such as danusertib, which showed activity in a small. trial (EudraCT number 2007-004070-18)17 but has no further ongoing trials in CML. Clinical trials in CML (NCT00405054; NCT00500006) of another pan-aurora kinase inhibitor, tozasertib (MK-0457), were terminated, in part because the agent showed only minimal efficacy even at high doses with intolerable toxicity.18
Other agents under investigation include the PPAR-g agonist pioglitazone (Actos), which is approved for treatment of diabetes. In the ACTIW trial, patients with CML-CP are randomized to either pioglitazone or the PD-L1 inhibitor avelumab (Bavencio), taken sequentially or in combination with 1 of several TKIs (NCT02767063). Vildagliptin (Galvus), another antidiabetic agent, is being investigated in patients taking nilotinib as pretreatment prior to attempting discontinuation of nilotinib (EudraCT: 2017-000899-28).5
Looking Ahead
Cortes said he expects asciminib to be approved initially for use in patients with CML after the failure of at least 2 TKIs, noting promising data in patients with the T315I mutation. However, he did point out that patients with this mutation were excluded from the phase 3 ASCEMBL study because the comparator, bosutinib, is inactive against this mutation.3 He said he hoped asciminib would be considered for approval for treating this mutation because “in that context, we only have 1 drug, ponatinib. Even putting aside the issues of safety, having 1 drug only form any given scenario is never a good idea.”
Regarding asciminib’s potential in an earlier setting, Cortes said, “it’s likely that it would work better in the second line than any of the other drugs that we [currently have].”
When asked about future development of targeted agents for patients with CML, Cortes said there is an unmet need due to a lack of effective therapies for blast-phase CML. “We don’t have good outcomes in blast-phase [disease]…but certainly, the need is not as immediate as it is for patients [with CML-CP] who have [prior treatment failure with] 2 TKIs or more, or patients who have the T315I [mutation].”
References:
1. NCCN. Clinical Practice Guidelines in Oncology. Chronic myeloid leukemia, version 2.2021. Accessed August 28, 2020. https://bit.ly/34AY9u4
2. Iclusig. Prescribing information. Takeda Pharmaceutical Company Limited; 2020. Accessed October 6, 2020. https://bit.ly/34tPDMe
3. Mauro MJ, Hochhaus A, Boquimpani C, et al. A multicenter, randomized phase III study of asciminib (ABL001) versus bosutinib in patients (pts) with chronic myeloid leukemia in chronic phase (CML-CP) previously treated with ≥ 2 tyrosine kinase inhibitors (TKIs). J Clin Oncol. 2019;37(suppl 15):TPS7070. doi:10.1200/JCO.2019.37.15_suppl.TPS7070
4. Carofiglio F, Trisciuzzi D, Gambacorta N, Leonetti F, Stefanachi A, Nicolotti O. Bcr-Abl allosteric inhibitors: where we are and where we are going to. Molecules. 2020;25(18):E4210. doi:10.3390/molecules25184210
5. Westerweel PE, Te Boekhorst PAW, Levin MD, Cornelissen JJ. New approaches and treatment combinations for the management of chronic myeloid leukemia. Front Oncol. 2019;9:665. doi:10.3389/fonc.2019.00665
6. Manley PW, Barys L, Cowan-Jacob SW. The specificity of asciminib, a potential treatment for chronic myeloid leukemia, as a myristate-pocket binding ABL inhibitor and analysis of its interactions with mutant forms of BCR-ABL1 kinase. Leuk Res. 2020;98:106458. doi: 10.1016/j.leukres.2020.106458
7. Novartis investigational novel STAMP inhibitor asciminib (ABL001) meets primary endpoint of phase III chronic myeloid leukemia study. News release. Novartis. August 26, 2020. Accessed August 26, 2020. https://bit.ly/2SAuwC8
8. Eide CA, Zabriskie MS, Savage Stevens SL, et al. Combining the allosteric inhibitor asciminib with ponatinib suppresses emergence of and restores efficacy against highly resistant BCR-ABL1 mutants. Cancer Cell. 2019;36(4):431-443.e5. doi:10.1016/j.ccell.2019.08.004
9. Jones JK, Thompson EM. Allosteric inhibition of ABL kinases: therapeutic potential in cancer. Mol Cancer Ther. 2020;19(9):1763-1769. doi:10.1158/1535-7163.MCT-20-0069
10. Hughes TP, Mauro MJ, Cortes JE, et al. Asciminib in chronic myeloid leukemia after ABL kinase inhibitor failure. N Engl J Med. 2019;381(24):2315-2326. doi:10.1056/NEJMoa1902328
11. Rea D, Lang F, Kim DW, et al. Asciminib, a specific allosteric BCR-ABL1 inhibitor, in patients with chronic myeloid leukemia carrying the T315I mutation in a phase 1 trial. Blood. 2018;132(suppl 1):792. doi:10.1182/blood-2018-99-113609
12. Hughes T, Mauro MJ, Kim DW, et al. Asciminib in heavily pretreated patients (pts) with Philadelphia chromosome–positive (Ph+) chronic myeloid leukemia in chronic phase (CML-CP) sensitive to tyrosine kinase inhibitor (TKI) therapy. Presented at: 25th European Hematology Association Annual Congress; June 11-21, 2020; Virtual. Abstract S170. Accessed October 22, 2020. https://bit.ly/30CTbe3
13. Cortes J, Lang F, Kim DW, et al. Combination therapy using asciminib plus imatinib (IMA) in patients (pts) with chronic myeloid leukemia (CML): results from a phase 1 study. Presented at: 25th European Hematology Association Annual Congress; June 11-21, 2020; Virtual. Abstract S883. Accessed October 22, 2020. https://bit.ly/35mefXP
14. Mauro MJ, Kim DW, Cortes J, et al. Combination of asciminib plus nilotinib (NIL) or dasatinib (DAS) in patients (pts) with chronic myeloid leukemia (CML): results from a phase 1 study. Presented at: 25th European Hematology Association Annual Congress; June 11-21, 2020; Virtual. Abstract S884. Accessed October 22, 2020. https://bit.ly/3lkFWXb
15. Cortes J, Jabbour E, Daley GQ, et al. Phase 1 study of lonafarnib (SCH 66336) and imatinib mesylate in patients with chronic myeloid leukemia who have failed prior single-agent therapy with imatinib. Cancer. 2007;110(6):1295-1302. doi:10.1002/cncr.22901
16. Cortes J, Quintás-Cardama A, Garcia-Manero G, et al. Phase 1 study of tipifarnib in combination with imatinib for patients with chronic myelogenous leukemia in chronic phase after imatinib failure. Cancer. 2007;110(9):2000-2006. doi:10.1002/cncr.23006
17. Borthakur G, Dombret H, Schafhausen P, et al. A phase I study of danusertib (PHA-739358) in adult patients with accelerated or blastic phase chronic myeloid leukemia and Philadelphia chromosome-positive acute lymphoblastic leukemia resistant or intolerant to imatinib and/or other second generation c-ABL therapy. Haematologica. 2015;100(7):898-904. doi:10.3324/haematol.2014.115279
18. Seymour JF, Kim DW, Rubin E, et al. A phase 2 study of MK-0457 in patients with BCR-ABL T315I mutant chronic myelogenous leukemia and Philadelphia chromosome-positive acute lymphoblastic leukemia. Blood Cancer J. 2014;4(8):e238. doi:10.1038/bcj.2014.60
