Combinations Offer Multiple Treatment Options in Relapsed/Refractory Multiple Myeloma
A 78-year-old White woman was given a diagnosis of stage II multiple myeloma and was deemed transplant ineligible. Genetic testing showed deletion 17p.
Joshua Richter, MD

During a Targeted Oncology™ Case-Based Roundtable event, Joshua Richter, MD, assistant professor at The Tisch Cancer Institute Icahn School of Medicine at Mount Sinai and director of Multiple Myeloma at the Blavatnik Family-Chelsea Medical Center at Mount Sinai, discussed the case of a 78-year-old woman with multiple myeloma.

Targeted OncologyTM: What FDA-approved regimens are preferred by the National Comprehensive Care Network (NCCN) for the treatment of relapsed/refractory multiple myeloma (RRMM)?
RICHTER: [When considering] the list of the FDA-approved, NCCN-preferred regimens for RRMM, something [to note] is that the combination of carfilzomib [Kyprolis], pomalidomide [Pomalyst], and dexamethasone is not FDA approved.1 It was studied in phase 2 [and phase 3] studies [NCT00603447 and NCT1080391, respectively] but not FDA approved.2,3 That being said, the combination of bortezomib [Velcade], lenalidomide, and dexamethasone, [which does appear on the list], was not a phase 3-studied regimen until a few years ago [NCT00644228], and we’ve all been using it for a decade.1,4 This combination has fallen out of favor, especially because so many patients progress on lenalidomide in the first line.
A number of daratumumab-based regimens are listed as well, combining daratumumab plus dexamethasone with bortezomib, with carfilzomib, or with lenalidomide.1 We don’t yet understand the role of daratumumab sequencing; although I don’t treat lymphoma, I hear you can [continue] the rituximab [Rituxan] along the way and [additionally] give cyclophosphamide, doxorubicin, vincristine, and prednisone; ifosfamide, carboplatin, and etoposide; or dexamethasone, high-dose cytarabine, and cisplatin.
There are also some rare [combinations] listed, like the ixazomib [Ninlaro], lenalidomide, dexamethasone combination, and the pomalidomide [Pomalyst], bortezomib, dexamethasone combination, especially [useful] for a patient who is proteasome inhibitor [PI] naive. Most of these combinations, preferred by the NCCN for previously treated myeloma, are supported by category 1 data. This is a moving point. Most patients get bortezomib [as first-line therapy these days]: cyclophosphamide, bortezomib, and dexamethasone; or bortezomib, lenalidomide, and dexamethasone, with or without daratumumab. However, that’s about to change. Now we have daratumumab plus lenalidomide starting to [replace] some of that treatment, and we have patients entering the second line completely [PI] naive. [Incorporating] a PI [like bortezomib] is a great approach from this standpoint.5 [Identifying the correct partner drug] is more complicated, but using a PI is a great way to go.
What do the European Hematology Association (EHA) and the European Society for Medical Oncology (ESMO) have to say about second-line options for RRMM following treatment with daratumumab, lenalidomide, and dexamethasone?
This is a new group of patients that we haven’t dealt with before. If the patient is lenalidomide refractory, the EHA-ESMO recommends dexamethasone with 1 of the following: pomalidomide plus bortezomib, selinexor [Xpovio] plus bortezomib, or carfilzomib. They also recommend venetoclax [Venclexta], bortezomib, and dexamethasone for patients with translocation (11;14). There are [additional] options for a patient who is lenalidomide sensitive, having stopped the lenalidomide, and then either stopping the daratumumab as well then progressing, or continuing on daratumumab alone [and then progressing]. For patients who are lenalidomide sensitive, EHA-ESMO additionally recommends the elotuzumab [Empliciti], lenalidomide, and dexamethasone combination or the carfilzomib, lenalidomide, dexamethasone combination.6
Can you summarize the bortezomib-containing options for RRMM?
Bortezomib plus dexamethasone, with and without daratumumab, [was examined in] the CASTOR study [NCT02136134] [median progression-free survival (PFS), not reached vs 7.2 months, respectively; HR, 0.39; 95% CI, 0.28-0.53; P < .001].7 The elotuzumab, bortezomib, and dexamethasone combination never went very far [NCT01478048] [median PFS, 9.7 months for the elotuzumab-containing triplet vs 6.9 months for the doublet without elotuzumab; HR, 0.72; 70% CI, 0.59-0.88; stratified log-rank P = .09].8 That triplet is not an approved regimen. Bortezomib plus dexamethasone, with and without pomalidomide, was examined in the OPTIMISMM study [NCT01734928] [median PFS, 11.20 months vs 7.10 months, respectively; HR, 0.61; 95% CI, 0.49-0.77; P < .0001].9 Bortezomib plus dexamethasone, with and without panobinostat [Farydak], was examined in the PANORAMA studies, more recently in the PANORAMA 3 study [NCT01023308] [median PFS, 11.99 months vs 8.08 months, respectively; HR, 0.63; 95% CI, 0.52-0.76; P < .0001].10 Finally, there was the BOSTON study [NCT03110562], comparing bortezomib plus dexamethasone with and without selinexor [median PFS, 13.93 months vs 9.46 months, respectively; HR, 0.70; 95% CI, 0.53-0.93; P = .0075].11 The median number of prior lines of therapy and the cytogenetic risk of the patients were different [among the studies]; it’s hard to [make] cross-trial comparisons. The way I approach it is [to ask what the patient is] refractory to and what mechanisms of action they are naïve or intolerant to. Consider whether you would feel differently about using another immunomodulatory imide drug [IMiD] at the time of relapse if the patient progressed after being on a lenalidomide-based regimen for 3 months vs 20 months.
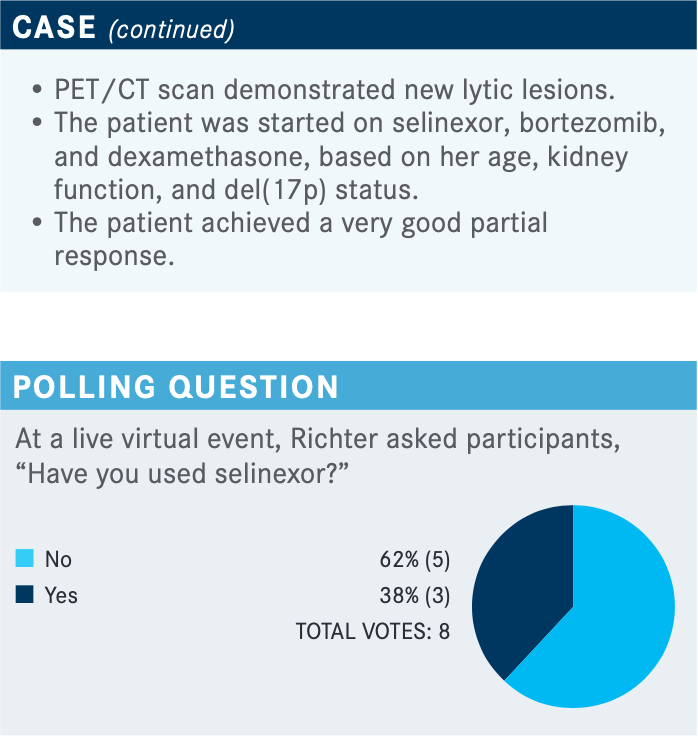
What are the data behind using selinexor for this patient?
The original FDA approval of selinexor for multiple myeloma was [based on] the STORM study [NCT02336815].12,13 But in December of last year, the [FDA approved selinexor for RRMM14 based on the] BOSTON study. The BOSTON study was done in patients with 1 to 3 prior lines of therapy.11 It was a very different approach. [Currently], most people do not get selinexor in early lines of therapy, but I think more patients [are going to] enter the second line already refractory to an IMiD, a PI, or a monoclonal antibody, especially as quadruplet regimens like daratumumab, lenalidomide, bortezomib, and dexamethasone are being used up front. [We are going to] have to look at some of the newer [options] like selinexor, idecabtagene vicleucel [Abecma], and belantamab mafodotin-blmf [Blenrep].
How was the BOSTON study designed and what were the outcomes?
The BOSTON study was a head-to-head study. In the bortezomib-dexamethasone [doublet] arm, [they used] the old-fashioned 3-week schedule: 2 weeks on, 1 week off, with bortezomib at 1.3 mg/m2 given on days 1, 4, 8, and 11. Dexamethasone at 20 mg was given the day of and the day after [each bortezomib dose]. The BOSTON group still believes giving steroids the day after bortezomib will reduce the incidence of neuropathy. This regimen was compared with weekly selinexor at 100 mg, weekly bortezomib at 1.3 mg/m2, and dexamethasone at 20 mg given the day of and the day after [each bortezomib dose].11
The PFS in the intention-to-treat arm was improved, as described above.11 [Additionally], we see that certain subgroups did particularly well, including the group with del(17p) [HR, 0.38; 95% CI, 0.16-0.86]—the same deletion found in the patient we are considering here. I feel that, for patients with particularly bad disease, bringing in [drugs with] new mechanisms of action [MOAs] at each relapse is key. Every drug doesn’t have to [represent] a new MOA; [after] daratumumab, lenalidomide, bortezomib, and dexamethasone, you can [switch] to something like carfilzomib and pomalidomide, where you’re [introduc-ing] a PI [and a] second-generation IMiD. But depending upon [the patient’s response, sometimes switching to new MOAs for the entire regimen] makes more sense. [For example], if you have a short-duration response on daratumumab and lenalidomide, [switching] to something like selinexor, bortezomib, and dexamethasone may make a lot more sense.
[Another BOSTON study subgroup that showed promising results consisted of those] with compromised renal function; patients with an estimated creatinine clearance rate of 30 to 60 ml/min showed significant improvement [HR, 0.49; 95% CI, 0.27-0.89].11 Part of this has to do with [the fact that] patients with poor renal function do worse, and better disease control provides better outcome.
The overall response rates were 76.4% for the triplet arm and 62.4% for the doublet arm [odds ratio, 1.96; 96% CI, 1.3-3.1; P = .0012], with a median time to response of 1.1 months [interquartile range (IQR), 0.8-1.6] vs 1.4 months [IQR, 0.8-1.6], respectively, and a median duration of response of 20.3 months vs 12.9 months, respectively [HR, 0.81; 95% CI, 0.56-1.17; P = .1364].11
No one is shocked that a 3-drug regimen [was superior to] a 2-drug regimen, but I think that [this triplet] is a great option [for previously treated multiple myeloma], even though quadruplets [like daratumumab, lenalidomide, bortezomib, and dexamethasone] are used [in the first line].
Nobody continues the quadruplet to progression; that’s not economically feasible, and it’s rough on the patient. Most patients go from [that quadruplet] to something like daratumumab and lenalidomide, then the patient is progressing in the second line, still sensitive to bortezomib. Bortezomib, pomalidomide, and dexamethasone [would not be] wrong there, but sometimes bringing in a new MOA, like selinexor or 1 of the other new drugs, is worthwhile to consider.
What was the toxicity profile of this trial like for patients?
Regarding adverse events [AEs], we’re going to have a higher rate of grade 3 and grade 4 hematologic AEs with a selinexor-based regimen [vs the doublet with no selinexor]. These rates were 39% vs 17%, respectively, for thrombocytopenia and 16% vs 10%, respectively, for anemia.11 One of the advantages of giving selinexor [only] once weekly has to do with the platelets. A selective inhibitor of nuclear transport, selinexor [has an affinity for] megakaryocytes and [decreases] platelet count [by] blocking XPO1, which has some homology with blocking thrombopoietin [TPO], part of the platelet pathway. If you give selinexor twice a week, TPO mimetics like romiplostim [Nplate] or eltrombopag [(Promacta) are not sufficient to counter selinex-or’s antiplatelet effect]. However, when you give selinexor [only] once weekly, TPO mimetics [can be effective; this is useful in] the later lines, when the patients are more [frail] and their marrow [is weaker].
Another thing [I want to mention] is that I think we all have concern for [persons 65 years and older]. For the older, frailer patients, we wonder whether we can use a triplet. [In my opinion], it’s better to give a dose-adjusted triplet than a full-dose doublet, [and patients in this age category showed a positive response to the triplet: HR, 0.55; 95% CI, 0.37-0.83].11
What monitoring and supportive care should be provided to patients being treated with selinexor?
Monitoring should include standard blood chemistry tests and assessments of complete blood cell count, weight, [circulatory] volume, and nutrition. [Patients can be given] aggressive treatment with antiemetics and intravenous fluid as needed.15 We have [seen] a lot of benefit using olanzapine [Zyprexa] at a low dose, 2.5 to 7.5 mg. We have also had success with rolapitant [Varubi], a [neurokinin-1] receptor antagonist, like aprepitant [Emend], except it’s oral and has fewer drug-drug interactions with selinexor. We administer rolapitant as two 90-mg tablets every 2 weeks. My preferred [supportive care combination] is ondansetron [Zofran], rolapitant, and dexamethasone, which is part of the regimen.
What are your thoughts about selinexor dosing?
Although cross-trial comparisons are difficult to make, comparing the AE profile of the original STORM study with that of the BOSTON study gives us some insight into the better tolerability of the once-weekly dosing [used in the BOSTON study vs the biweekly dosing used in the STORM study].11,13 With respect to grade 3 and grade 4 AEs, the percentage of patients with thrombocytopenia went from 58% to 39%, neutropenia [dropped to] the single digits [from 21% to 9%], and hyponatremia and fatigue were cut almost in half [to 14% and to 13%, respectively]. In general, this is a far better regimen, albeit [used in an] earlier line, which definitely [matters], but far better tolerated in terms of hematologic AEs and some nonhematologic AEs as well.
I don’t give twice-weekly selinexor to anyone. For most people, I start with 100 mg once weekly. Soon I’ll be starting a patient on selinexor and isatuximab-irfc [Sarclisa]. This patient is in their 80s and has renal insufficiency and another metastatic cancer, so I will start the selinexor at 80 mg once weekly. But in general, [selinexor can be started at] 100 mg once weekly, followed by reductions to 80 mg, 60 mg, and 40 mg.15 I don’t give anyone 80 mg twice weekly anymore.
There are additional dose modification guidelines that specifically address hematologic AEs. For the patients who are otherwise doing well, if the platelet count gets low or the patient becomes significantly neutropenic, you have to drop a dose level.15 If [a patient starts] a regimen, and their marrow has already been obliterated because of marrow infiltration by plasma cells, you have to treat through.
REFERENCES
1. NCCN. Clinical Practice Guidelines in Oncology. Multiple myeloma, version 1.2022. Accessed September 14, 2021. https://bit.ly/3ltdrZ6
2. Wang M, Martin T, Bensinger W, et al. Phase 2 dose-expansion study (PX-171-006) of carfilzomib, lenalidomide, and low-dose dexamethasone in relapsed or progressive multiple myeloma. Blood. 2013;122(18):3122-3128. doi:10.1182/blood-2013-07-511170
3. Stewart AK, Rajkumar SV, Dimopoulos MA, et al. Carfilzomib, lenalidomide, and dexamethasone for relapsed multiple myeloma. N Engl J Med. 2015;372(2):142-152. doi:10.1056/NEJMoa1411321
4. Durie BGM, Hoering A, Abidi MH, et al. Bortezomib with lenalidomide and dexamethasone vs lenalidomide and dexamethasone alone in patients with newly diagnosed myeloma without intent for immediate autologous stem-cell transplant (SWOG S0777): a randomised, open-label, phase 3 trial. Lancet. 2017;389(10068):519-527. doi:10.1016/S0140-6736(16)31594-X
5. Velcade. Prescribing information. Takeda; 2019. Accessed September 16, 2021. https://bit.ly/39pNe8b
6. Dimopoulos MA, Moreau P, Terpos E, et al; European Hematology Association Guidelines Committee; European Society for Medical Oncology Guidelines Committee. Multiple myeloma: EHA-ESMO clinical practice guidelines for diag-nosis, treatment and follow-up. Ann Oncol. 2021;32(3):309-322. doi:10.1016/j.annonc.2020.11.014
7. Palumbo A, Chanan-Khan A, Weisel K, et al; CASTOR Investigators. Daratumumab, bortezomib, and dexamethasone for multiple myeloma. N Engl J Med. 2016;375(8):754-766. doi:10.1056/NEJMoa1606038
8. Jakubowiak A, Offidani M, Pégourie B, et al. Randomized phase 2 study: elotuzumab plus bortezomib/dexamethasone vs bortezomib/dexamethasone for relapsed/refractory MM. Blood. 2016;127(23):2833-2840. doi:10.1182/blood-2016-01-694604
9. Richardson PG, Oriol A, Beksac M, et al; OPTIMISIMM Trial Investigators. Pomalidomide, bortezomib, and dexamethasone for patients with relapsed or refractory multiple myeloma previously treated with lenalidomide (OPTIMISMM): a randomised, open-label, phase 3 trial. Lancet Oncol. 2019;20(6):781-794. doi:10.1016/S1470-2045(19)30152-4
10. San-Miguel JF, Hungria VT, Yoon SS, et al. Panobinostat plus bortezomib and dexamethasone vs placebo plus bortezomib and dexamethasone in patients with relapsed or relapsed and refractory multiple myeloma: a multicentre, randomised, double-blind phase 3 trial. Lancet Oncol. 2014;15(11):1195-1206. doi:10.1016/S1470-2045(14)70440-1
11. Grosicki S, Simonova M, Spicka I, et al. Once-per-week selinexor, bortezomib, and dexamethasone vs twice-per-week bortezomib and dexamethasone in patients with multiple myeloma (BOSTON): a randomised, open-label, phase 3 trial. Lancet. 2020;396(10262):1563-1573. doi:10.1016/S0140-6736(20)32292-3
12. FDA grants accelerated approval to selinexor for multiple myeloma. FDA. July 3, 2019. Accessed September 15, 2021. https://bit.ly/39izw6R
13. Chari A, Vogl DT, Gavriatopoulou M, et al. Oral selinexor-dexamethasone for triple-class refractory multiple myeloma. N Engl J Med. 2019;381(8):727-738. doi:10.1056/NEJMoa1903455
14. FDA approves selinexor for refractory or relapsed multiple myeloma. FDA. December 18, 2020. Accessed September 15, 2021. https://bit.ly/3Evjt4f
15. Xpovio. Prescribing information. Karyopharm Therapeutics Inc; 2020. Accessed September 16, 2021. https://bit.ly/39eSZFB

Bispecific Antibodies and ADCs Deliver a Futuristic Horizon Across Lung Cancer Settings
October 23rd 2024Recent advancements in protein engineering, especially antibody-drug conjugates, show promise in lung cancer treatment, with ivonescimab outperforming pembrolizumab in PD-L1-positive advanced non-small cell lung cancer.
Read More