Considering Outpatient Use of Tagraxofusp for BPDCN in the Community Setting
During a Targeted Oncology™ Case-Based Roundtable™ event, Jonathan Abbas, MD, discussed with other oncologists concerning the practical considerations for outpatient use of tagraxofusp-erzs for patients with blastic plasmacytoid dendritic cell neoplasm.
Jonathan Abbas, MD (MODERATOR)
Medical Director, Ascension Saint Thomas Blood Cancer Program
Tennessee Oncology Midtown Center for Blood Cancers
Nashville, TN

PARTICIPANT LIST: Roger Fleischman, MD | Jeremy Pantin, MD | Jack Erter, MD | Chirag Amin, DO | Daniel Vaena, MD
Event Region: INDIANA, KENTUCKY, TENNESSEE
CASE SUMMARY
A man aged 67 years was referred from a dermatologist. He was referred initially to the dermatologist by his primary care physician for progressive, persistent, cutaneous nodules that the patient had noticed 3 weeks prior. The patient had fatigue and 5-kg (11-lb) weight loss over 3 months, and a medical history of sinusitis but no major comorbidities. Upon physical examination, there were multiple purpuric nodules (measuring up to 5 cm on arms, legs, torso). No palpable adenopathy or hepatosplenomegaly was observed. His ECOG performance status was 1.
Laboratory results:
- White blood cell count: 14.1 × 10³/μL
- Hemoglobin: 8.9 g/dL
- Platelets: 54 × 10³/μL
- Differential revealed 12% blasts, 32% neutrophils, 16% monocytes, 40% lymphocytes
The patient’s skin had a purpuric nodule. On a peripheral blood smear, there were blastic cells with large and round or slightly irregular nuclei; blast cytoplasm stained grayish blue without granules or Auer rods.
A bone marrow biopsy showed 40% blasts by morphology; 80% cellular marrow with interstitial infiltrate. Immunohistochemistry of neoplastic cells showed the patient was CD123, CD4, CD56, and TCL1 positive. Flow cytometry showed that CD4, CD56, CD123, CD34, and T-cell and B-cell lineage-specific markers were negative. Lumbar puncture did not indicate central nervous system involvement. The patient was diagnosed with blastic plasmacytoid dendritic cell neoplasm (BPDCN) based on clinical and histopathological findings.
DISCUSSION QUESTION
- How would you approach administering tagraxofusp-erzs (Elzonris) to patients with BPDCN?
FLEISCHMAN: Would you consider initiating therapy as an outpatient?
ABBAS: It depends on the patient. All my cases preceded the use of this [therapy], but when they were doing this on trial in Houston, [at The University of Texas MD Anderson Cancer Center], they wanted them there for a month, but they were administering it in the outpatient setting.
FLEISCHMAN: [But that] is MD Anderson’s outpatient setting. It’s not my outpatient setting.
ABBAS: Yes, so my take on it would be I would have no qualms about doing this in the outpatient setting, but I would be checking my laboratory results every day. I would be making sure I was ready to infuse albumin. It depends on the age of the patients and degree of organ involvement. A [patient aged 70 years] with widespread visceral disease is different than a [patient aged 30 years] with skin-only involvement.
I don’t know how well the degree of involvement correlates to the rate of toxicity of capillary leak syndrome [CLS]. I couldn’t find anything to say the higher disease burden has a higher risk of CLS. From a logical standpoint, patients with higher disease burden are going to be a little more fragile.
Those would be the patients I’d be worried about. I do not know about the finances of this drug, but inpatient administration would have some potentially significant financial toxicity. We obviously [must] do what we have to do, but it certainly seems like trying to give this in the outpatient setting, being ready to jump in with inpatient support as needed, is the optimal way to go.
FLEISCHMAN: It’s given 5 days in a row every 21 days?1
ABBAS: Correct.
FLEISCHMAN: When in the 21- day cycle could you have CLS? Immediately, of course, but [could it also be] 10 or 12 days out?
ABBAS: I did find…that it can be variable [median time to onset, 4 days (range, 1-46)].1,2 That is why you have to keep monitoring these patients multiple times a week, especially all the way through that first cycle. The time to response is so fast [that] these patients go into a remission within a cycle or so. It’s not like you must be worrying on cycle 8 of this drug [if ] someone is going to get CLS.
I’m sure it’s theoretically possible, [but] they probably wouldn’t. I would set it up as 1 month of intense monitoring and within a month or so you should know your response.
FLEISCHMAN: It’s not going to be good for patients to drive 2 hours each way to see you.
ABBAS: No, it’s not. For those patients, you might just do what we do. We have patients with acute myelocytic leukemia for whom we give a hypomethylator/ venetoclax [Venclexta] induction.
They live 2 hours away, so we drop them in the hospital and they [must] spend a month in the hospital for an “outpatient regimen,” yet you do what you [must] do. My referral basis is 2.5 to 3 hours away.
PANTIN: Dr Abbas, at the time it was released, [tagraxofusp] was $25,000 for 1 vial.
ABBAS: There are more expensive [therapies] out there. If you can make a vial [that can] get you through several doses, that’s better than I thought it was going to be. The principle of supportive care [includes monitoring of] albumin…. You want albumin [3.2 g/dL] to be considered an acceptable baseline. As soon as you see a reduction of 0.5 g/dL from baseline, you want to be stepping in with albumin replacement and you want [premedication with H1 or H2] histamine antagonist, steroids, [etc].
If you ever were going to give this in your clinic, you want to be educating your nursing staff about what worst-case scenarios…could look like.
There is a nice list of reasons to hold. I was happy with the NCCN [National Comprehensive Cancer Network] guidelines on this. I thought they were thorough and made it…clear as when to start worrying yourself [Figure3].
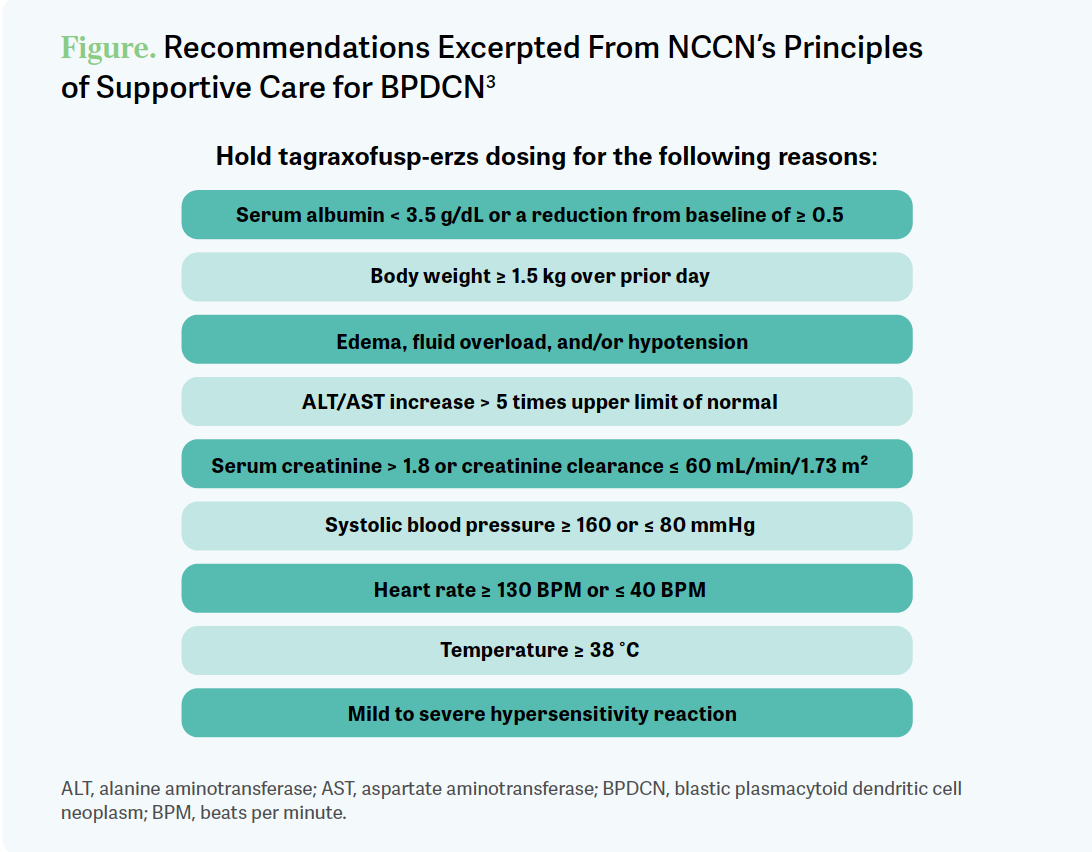
ABBAS: I think myself, Dr Pantin, and [some other participants]…would be comfortable administering this in our clinic with the level of supportive care we have. [For] some of the other [participants], is this the kind of thing you would think about giving in your clinic or was this the kind of thing… you want to send to us [at Tennessee Oncology in Nashville]?
ERTER: With the CLS I would…want to, at the very least, get a patient started with [a tertiary care center]. I do a clinic currently in McMinnville, [Tennessee], once a week, so those patients have a hard time getting to Nashville for extended periods…. I would prefer to send it just from a toxicity management standpoint, but I would also be open to getting some guidance.
AMIN: I also do a satellite office…and I think the same thing. The first month would have to be with [a tertiary care center] because we just don’t have the laboratory or any of the supportive measures. After that, you’d have to have a little bit of a learning curve, but I could [give treatment to] them somewhere else.
ABBAS: I agree, and there are enough similarities [ for participants in] Kentucky where I’m guessing...we’d all be in the same sort of boat.
VAENA: If I had a patient like this, I’d be sending them to one of our hematologists. For the patients who are getting the drug alone without expectation for transplant, I saw the relapse rates after a year or 2. Is it a given that everybody will relapse given enough time? [Did] patients come off for progression or did patients mostly come off for toxicity?
ABBAS: On the clinical trial, virtually everybody who did not go on to an allogeneic stem cell transplant stayed on it until progression.2 The only long-term survivors there were patients who did go on to allogeneic transplant.
Even if you do achieve a CR quickly as a lot of patients might, most patients who achieved a CR stayed on until progression or toxicity, but [most] of them were relapsing within a year.
MODERATOR SIDEBAR
Targeted Oncology: What stood out in terms of toxicity of tagraxofusp for blastic plasmacytoid dendritic cell neoplasm in the phase 2 trial (NCT02113982)?
ABBAS: Looking at adverse events…the main ones of higher grades to look for are LFT [liver function test] increases—[patients require] routine monitoring of LFTs—and thrombocytopenia [Table2]. The big ones to look for leading to discontinuation are weight gain, LFTs, and then the hypoalbuminemia where CLS [capillary leak syndrome] comes up for some patients.
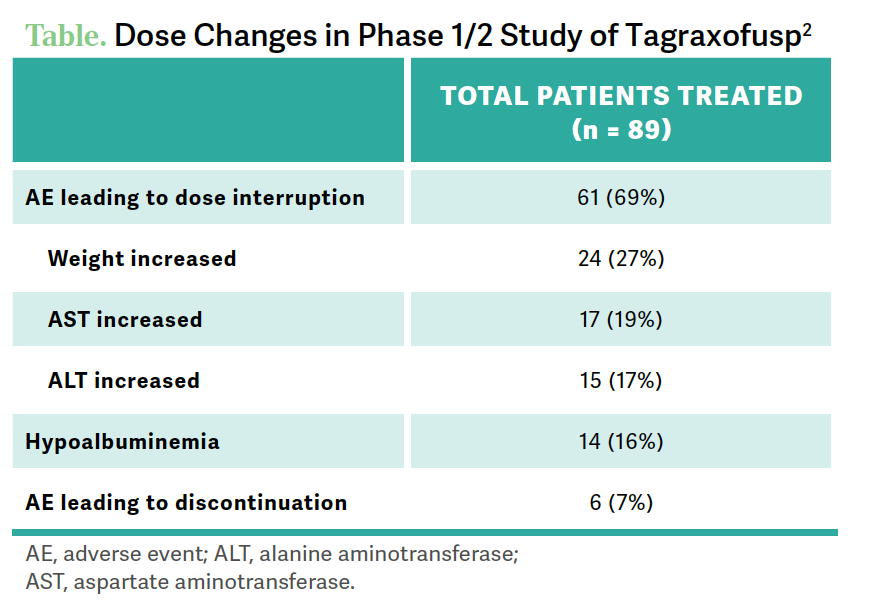
[For] CLS, half the patients [46%] had [low] grade.1 There were some more advanced grades seen [6% grade 3 and 1% grade 4], but there were 2 fatal events [in] this [cohort]… out of a small group. That’s why we [must] take this very seriously.
How should physicians manage risk of CLS?
Signs and symptoms of CLS [are] low albumin, which we’re monitoring and replacing regularly, edema, weight gain, and hypotension.
As a management algorithm, make sure you have a good baseline serum albumin level. [Have] a low threshold to replace it, to administer intravenous albumin as often as necessary. If this could be managed in the outpatient setting, [that would be] great. If it requires hospitalization, so be it.
Monitor daily weight, use of steroids, use of things like pressors if needed. These patients could potentially get critically ill, so it is absolutely something to keep an eye on and you want to be administering this if you’re going to take it upon yourself to do this, even though we can give the drug easily in clinic.
You want to make sure that whatever hospital you’re using to support it can do it. That is why we see so many tertiary referrals for this kind of thing. If these patients are going to go bad, we want them to be getting this level of borderline [intensive care unit] care in a center that has good experience with hematologic malignancies and supporting patients through neutropenic sepsis and leukemia inductions, etc.
REFERENCES
1. Elzonris. Prescribing information. Stemline Therapeutics; 2022. Accessed June 13, 2023. https://bit.ly/3P9EM2M
2. Pemmaraju N, Sweet KL, Stein AS, et al. Long-term benefits of tagraxofusp for patients with blastic plasmacytoid dendritic cell neoplasm. J Clin Oncol. 2022;40(26):3032-3036. doi:10.1200/JCO.22.00034
3. NCCN. Clinical Practice Guidelines in Oncology. Acute myeloid leukemia, version 3.2023. Accessed June 13, 2023. https://bit.ly/3PcwyY0
