Expert Discusses Genomic Testing and Trials Pertinent to Treating Prostate Cancer
During a Targeted Oncology Case-Based Peer Perspectives event, Arash Rezazadeh Kalebasty, MD, discussed genomic testing and evidence-based treatment of a 60-year-old male patient with prostate cancer.
Arash Rezazadeh Kalebasty, MD

During a Targeted Oncology Case-Based Peer Perspectives event, Arash Rezazadeh Kalebasty, MD, an associate professor in the Division of Hematology/Oncology, Department of Medicine and, Department of Urology at the UCI School of Medicine in Irvine, CA, discussed genomic testing and evidence-based treatment of a 60-year-old male patient with prostate cancer.
Targeted OncologyTM: Which trials have demonstrated the clinical significance of BRCA mutations in prostate cancer?
KALEBASTY: The TRITON2 [NCT02952534] trial looked at rucaparib [Rubraca] for metastatic castrate-resistant prostate cancer [mCRPC].1 Screening [for the trial included] identification of the deleterious somatic or germline alterations in HRR [homologous recombination repair] genes. So [this] includes BRCA1, BRCA2, ATM, CHEK2, [BARD1, BRIP1, CDK12, FANCA, NBN, PALB2, RAD51, RAD51B, RAD51C, RAD51D, RAD54L]. Key eligibility criteria were mCRPC; [an HRR gene] mutation; disease progression on a targeted therapy for prostate cancer; 1 prior taxane-based chemotherapy for CRPC; ECOG, 0 or 1; and no prior PARP inhibitor, mitoxantrone, cyclophosphamide, or platinum-based chemotherapy.
The treatment was rucaparib 600 mg twice daily. This was a phase 2 trial; [the investigators looked] at the tumor assessment every 8 weeks at the beginning and then it went to every 12 weeks. [They] continued treating [patients until] radiographic progression or discontinuation for other reasons.
Primary end point in patients with measurable disease at baseline was confirmed overall response rate by RECIST, [and in] patients with no measurable disease—which is common in prostate cancer, because a lot of times we get only bone metastases and no visceral metastases—at baseline we looked at PSA response. So if they had measurable disease it was by RECIST, if not the confirmed PSA response [of] more than 50% [decrease].
[More than] 1700 patients were screened and about 200 were enrolled. We had about 115 BRCA alterations identified in the screening. [At] data cutoff 29 patients remained on treatment— it was only 25% of patients. Median range of follow-up was 17 months.
Now, we looked at the germline [for 38.3% of patients]; it was 13 [total] patients [with] BRCA1; BRCA2 was [seen in] 100 patients. The BRCA1 is not a very common [mutation], BRCA2 was the main one in this group, but again, this is only for BRCA1 or BRCA2. The alteration of zygosity, in my opinion… is important. I’ve seen biallelic patients respond much better. Of course, if you have 1 good gene, it may be more difficult to hit it. For BRCA2, one-third of the patients were biallelic and a significant number of these patients [had] unknown zygosity [60.8%], so we don’t have the whole data because it wasn’t properly tested. But 33% is pretty good. I think that’s probably one of the reasons that we saw better response in this patient population.
Radiographic response for BRCA1 and BRCA2—this was restricted to this patient population—overall response rate was 43.5%. [There was] a PSA response rate of 54.8%, the stable disease was in 45.2%, and progressive disease was seen in 9.7% of patients to start with, so a majority of patients had a [clinical] benefit with CR [complete response] or PR [partial response] or stable disease.
The median radiographic progression-free survival [rPFS] was 9.0 months by blinded [review] and median overall survival [OS] was not mature—41% of events are reported and estimated 12-month OS [rate] was 73%. I think that that number by itself, if you treat prostate cancer, tells you something. I think that’s pretty good; after 2 lines of therapy in a castrate-resistant setting, you still get 73% OS [rate] at 12 months. Median time to PSA progression was 6.5 months.
Did response differ according to the different gene alterations studied?
The response by DDR [DNA damage repair] gene alteration [differed].2 If you look at the ATM [mutant group (n=49)] for CR, PR, and progressive disease, about 36% of patients had progressive disease to start with. [For] a majority of patients, if there was any benefit, it was of stable disease (47.4%), and CR or PR was not common; the [PR rate was] 10.5%.
For CDK12 [n=15], there was CR and PR of 0%; stable disease, about 60%; and progressive disease was 36.8%.
For CHEK2 [n=12]—which is another one that you see rather commonly compared with the others—there was a PR [of 11.1%] and stable disease of 66.7% and 22.2% with progressive disease.
So the point of this is if you look at the other mutations, other than BRCA1 and BRCA2, especially BRCA2, or non-BRCA, the data may not be as impressive, in my opinion. You don’t see that much of even PR, [with a PR rate of 21.4% as] a lump sum in the other mutations.
In my experience, PARP inhibitors…are pills that may not be as easy as androgen receptor target therapy. Fatigue was about 44%, nausea/vomiting, 42%, and there’s some vomiting, at 20%. There’s a lot of GI [gastrointestinal] issues [such as] constipation and diarrhea. Diarrhea is about 18%, 20% [had] ALT/AST [alanine/aspartate aminotransferase] increase. So you have to monitor the liver enzymes.
The FDA accelerated the approval for rucaparib based on phase 2 [data for patients with] BRCA-mutated mCRPC [who have been] treated with androgen receptor-directed therapy and a taxane-based chemotherapy.3 [The approval was for] patients with a BRCA mutation, either germline or somatic…so the other genes that they have tested basically are not included in the approval of this particular agent, although it was tested.
How do other studies compare with TRITON2?
The phase 3 PROfound [NCT02987543] study was with another PARP inhibitor, olaparib [Lynparza].4 Key eligibility criteria [included] mCRPC with disease progression on prior agent abiraterone [Zytiga] or enzalutamide [Xtandi] and an alteration in 1 or more qualifying gene alterations [that] have a direct or indirect role in HRR.
Cohort [A included alterations of] BRCA1, BRCA2, or ATM. Cohort B [included] other alterations. A majority of patients [were in] cohort A with 245 patients; they received olaparib 300 mg twice daily versus physicians’ choice for treatment. Both cohorts had a similar design of olaparib versus physicians’ choice, up until progression. Physicians’ choice patients were [able to] cross over to olaparib.
The primary end point was rPFS in cohort A, and a key secondary end point was rPFS in cohort A plus B. So they put everybody together, confirmed radiographic objective response rate in CT, time to pain progression in cohort A, and OS in cohort A, so the investigators picked certain things to look at as secondary end points in different cohorts.
[Of] the prespecified HRR-associated genes, an alteration in more than 1 of these genes was found in 28% of these 2800 samples. So, this is a significant number of alterations that were picked. But again, not every alteration is the same, not every alteration is as sensitive to the treatment.
What did the results of the PROfound study show?
The difference in median rPFS was 7.4 months with olaparib versus control of 3.6 months. The hazard ratio for progression or death was 0.34; it was a very high hazard ratio in my opinion, and P value was less than 0.001. The difference at 12 months and 6 months in rPFS is 60% versus 23% and 28% versus 9%, respectively. The probability of imaging-based PFS…was better in the treatment arm with olaparib.
In cohorts A plus B—the overall population—the median rPFS was 5.8 versus 3.5 months. And the hazard ratio was 0.49 with a P value of less than 0.001. Olaparib was significantly better for all-comers.
The subgroup populations showed significant improvement with olaparib; everything was [favoring olaparib]. I think the most important part of [the forest plot, of any] of the subgroups is BRCA and ATM. ATM passed 1.00, the ratio was 1.04. We have CDK12 and CHEK2 [below 1.00], and PPP2R2A [had a hazard ratio of 6.61]. Not every mutation is the same, but I have to admit the number of some of these mutations was not significant enough to really know what’s going on with them. But data are data and it does look like, at least based on this subgroup analysis, [that olaparib is] working with certain [alterations].
Cohort A…had a median OS of 19.1 months versus 14.7 months, a hazard ratio of 0.69, and a P value of 0.2.5
OS for the overall population—all-comers—[was 17.3 versus 14.0 months with olaparib versus control, respectively]. The hazard ratio was 0.79. Then if you adjust for cross over, the hazard ratio was 0.55.
Confirmed overall response in cohort A—again, this is for measurable disease—[was] 33% versus 2.3%; odds ratio for an objective response was 20.86. Though [it was] pretty significant.6
What was the safety profile like with olaparib? How did it compare with that of other PARP inhibitors?
The [rate of] dose reduction on olaparib versus patients on physicians’ choice was 22.3% versus 3.8%. Discontinuation due to AEs was 16.4% versus 8.5%, and death was 3.9% versus 3.8%. [It was] similar and reported to be related to this type of treatment. It’s about 1 [death] in each group.
PARP inhibitors sound good as an option, but they come with a cost, and one of the significant issues with these agents is bone marrow suppression and anemia; 46.5% of patients had that versus 15.4%. Nausea was 41.4% versus 19.2%; fatigue, 41.0% versus 32.3%; decreased appetite, 30.1% versus 17.7%; diarrhea is 21% versus 6.9%. These patients have some GI symptoms on PARP inhibitors, like nausea, vomiting, constipation, and lack of appetite.
How did the approval of olaparib compare with that of rucaparib?
The FDA approval of olaparib was for HRR gene–mutated mCRPC that progressed following prior treatment with enzalutamide or abiraterone.7 If [the patient] had chemotherapy it was allowed, but it wasn’t necessary to have failed chemotherapy. The difference between this and rucaparib is that rucaparib was only for BRCA mutations for HRR gene–mutated metastatic cancer, this [is for HRR gene mutations of any kind].
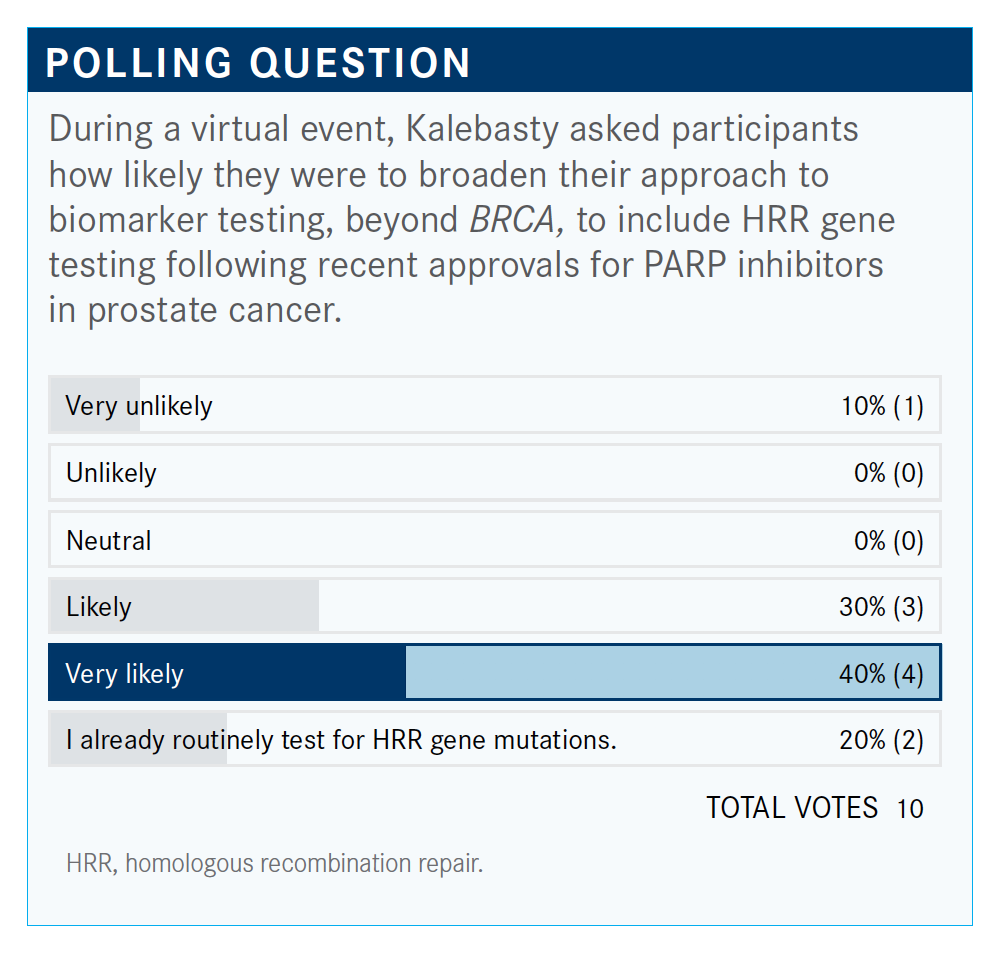
What are your thoughts on the results of the poll, and do you use broad HRR gene testing? If so, why?
Sometimes you can have these data and use it to enroll a patient in a clinical trial; for example, if there’s a new agent or new way. At least knowing what’s going on with these patients, the possibilities, sooner rather than later when you know the patient has a different biology and maybe there’s a different driver here—[so that you know you] need to pay attention, [and not to] expect for them to behave like other prostate cancers. So especially after failure back-to-back, you have a reason, now you know that there’s a driver there and maybe you have to act on it when you can. I understand that the drugs that we have may not be effective in certain mutations, but it doesn’t mean that there’s no ongoing effort to make it work somehow, whether it’s a combination with our new agents or whatnot.
Are there any issues with this testing in prostate cancer?
One of the problems with prostate cancer is running NGS [next-generation sequencing] based on bone biopsy, which is difficult and often you can’t run it. There are many reasons why. Sometimes you get soft tissue and send it if you have a lymph node or something that you can biopsy. But it is an issue, and we hope in the near future we have good enough liquid biopsies that we can use that to pick up the somatic mutations. For germ-line mutation, I believe that a liquid biopsy is pretty good, should be able to [pick it up] no problem. But for somatic, especially if you have not much of a tumor in your patient, it’s likely not to be very effective. But it sounds like the future is promising and, hopefully, we can be able to run liquid biopsies much more efficiently and more informatively.
How do you counsel patients who test positive for a non-BRCA HRR gene mutation?
For any [patients with] BRCA or non-BRCA HRR [mutations], it is so important to look for other possible primaries in the same patient [that] are associated with breast cancer, pancreatic cancer, and talk to them about that possibility. [It is] absolutely necessary to discuss the family members who may carry the gene; it’s a germline mutation. I often use the genetic counselor [because it is] so important to follow up in the future. Some of these mutations that are not relevant now may become relevant. So I think geneticists are playing an important role in making sure that the patient is followed up with properly. [In my part of the] counseling, I talk to the patient about the family members and about secondary cancers in themselves.
A lot of times we know these mutations at the time of diagnosis. For example, if a patient gets prostatectomy or gets radiotherapy to his prostate, and if this patient being tested is 45 years old with prostate cancer, those are the things that have to be followed. A lot of times we know these things because why would a patient [who is] 45 years old have prostate cancer? So those are the tests that we do and we discuss with them at that time. I test my patients, especially young patients with genetic testing. If you look at the National Comprehensive Cancer Network guidelines, [it says that] patients with CRPC should be tested for that, and this helps with their family members, but maybe [also for] younger patient with definitive treatment, it can be helpful for themselves.8
References:
1. Abida W, Patnaik A, Campbell D, et al; TRITON2 investigators. Rucaparib in men with metastatic castration-resistant prostate cancer harboring a BRCA1 or BRCA2 gene alteration. J Clin Oncol. Published online August 14, 2020. doi:10.1200/JCO.20.01035
2. Abida W, Campbell D, Patnaik A, et al. Non-BRCA DNA damage repair gene alterations and response to the PARP inhibitor rucaparib in metastatic castration-resistant prostate cancer: analysis from the phase 2 TRITON2 study. Clin Cancer Res. 2020;26(11):2487-2496. doi:10.1158/1078-0432.CCR-20-0394
3. FDA grants accelerated approval to rucaparib for BRCA-mutated metastatic castration-resistant prostate cancer. FDA. Updated May 15, 2020. Accessed November 17, 2020. https://bit.ly/2HGYMcy
4. de Bono J, Mateo J, Fizazi K, et al. Olaparib for metastatic castration-resistant prostate cancer. N Engl J Med. 2020;382(22):2091-2102. doi:10.1056/NEJMoa1911440
5. Hussain M, Mateo J, Fizazi K, et al; PROfound Trial Investigators. Survival with olaparib in metastatic castration-resistant prostate cancer. N Engl J Med. Published online September 20, 2020. doi:10.1056/NEJMoa2022485
6. Hussain M, Mateo J, Fizazi K, et al. PROfound: Phase 3 study of olaparib versus enzalutamide or abiraterone for metastatic castration-resistant prostate cancer (mCRPC) with homologous recombination repair (HRR) gene alterations. Ann Oncol. 2019;30(suppl 5):5059. doi:10.1093/annonc/mdz394
7. FDA approves olaparib for HRR gene-mutated metastatic castration-resistant prostate cancer. FDA. Updated May 20, 2020. Accessed November 25, 2020. https://bit.ly/3jIugw6
8. NCCN. Clinical Practice Guidelines in Oncology. Prostate Cancer, version 2.2020. Accessed October 30, 2020. https://bit.ly/37Ykhzd

Bispecific Antibodies and ADCs Deliver a Futuristic Horizon Across Lung Cancer Settings
October 23rd 2024Recent advancements in protein engineering, especially antibody-drug conjugates, show promise in lung cancer treatment, with ivonescimab outperforming pembrolizumab in PD-L1-positive advanced non-small cell lung cancer.
Read More