Gradishar Discusses Role of Elacestrant in ER+/HER2– Breast Cancer
During a Targeted Oncology™ Case-Based Roundtable™ event, William J. Gradishar, MD, discussed dosing and toxicity considerations related to elacestrant in metastatic breast cancer with participants.
William J. Gradishar, MD (MODERATOR)
Chief of Hematology and Oncology, Department of Medicine
Betsy Bramsen Professor of Breast Oncology
Northwestern University Feinberg School of Medicine
Chicago, IL

EVENT REGION Indiana and Kentucky
PARTICIPANT LIST Deng Zhang, MD, PhD | Karim Anwar, MD | Ajita Narayan, MD | Mohamed Khasawneh, MD | Bilal Siddiqui, MD
CASE SUMMARY
A 56-year-old postmenopausal woman presented with a palpable right breast mass with no clinically abnormal axillary lymph nodes. A core biopsy led to a diagnosis of grade 2, estrogen receptor– positive (ER+)/progesterone receptor–positive (PR+) invasive ductal carcinoma (IDC) with a HER2 immunohistochemistry (IHC) score of 0 and Ki-67 score of 33%. Lumpectomy and sentinel lymph node (SLN) biopsy results showed a tumor measuring 1.8 cm, grade 2 IDC, and 2 SLNs negative for malignant cells. Her 21-gene recurrence score was 27.
The patient received docetaxel (Taxotere) and cyclo-phosphamide (Cytoxan) for 4 cycles followed by radiation therapy, and she completed 5 years of adjuvant aromatase inhibitor (AI) therapy.
Three years later, she reported right-sided abdominal pain and mild nausea. A CT scan of the chest, abdomen, and pelvis showed 3 suspicious liver lesions (right lobe; largest measuring 2 cm). Liver biopsy results showed ER+/PR+ IDC with a HER2 IHC score of 0. Comprehensive molecular testing results from tissue biopsy showed no actionable alterations. AI therapy with letrozole (Femara) plus palbociclib (Ibrance) was initiated.
The therapy was well tolerated by the patient, with grade 2 neutropenia that did not require dose modification. Twenty months later, follow-up imaging showed enlargement of liver nodules and 2 new lung nodules (largest measuring 0.9 cm). Her ECOG performance status was 0. Blood-based circulating tumor DNA analysis showed an ESR1 mutation.

GRADISHAR: It looks like most people are choosing elacestrant [Orserdu] but exemestane [Aromasin] plus everolimus [Afinitor] was considered by at least 1 person, as well as fulvestrant [Faslodex]. I’d ask the person who chose monotherapy with fulvestrant [their reasoning], as well as any comment about those on the exemestane plus everolimus choice.
ZHANG: I chose fulvestrant; I think it’s a good medication. For [later] options, I think [targeting] the ESR1 mutation will potentially be stronger down the line so we can reserve the lines for later use. I still use [exemestane plus everolimus], and sometimes I can use it later even though the mucositis or adverse events [AEs] of everolimus are [concerning].
DISCUSSION QUESTION
- Have you used the oral SERD (selective estrogen receptor degrader) elacestrant for patients with breast cancer?
GRADISHAR: Has anybody had any experience with elacestrant at this point? What have you noted in terms of efficacy [and] tolerability?
ANWAR: I have 1 patient on elacestrant, and the patient is tolerating it well. It has been 2 months; it’s too early for me to tell about efficacy.
NARAYAN: I also have a patient [taking] elacestrant and she has been on it for 5 months. Her major problem with it seems to be fatigue; her fatigue is quite remarkable [because it] started approximately a few weeks after she began elacestrant. We are currently in the process of trying to work her up for other causes of fatigue.
GRADISHAR: Elacestrant is the only [oral SERD] that has been approved [by the FDA], but there are a lot of them that have been in clinical trials, and some of them have distinct AE profiles. We’ll see which ones, if any, get approved, but there are many in development at this point.
DISCUSSION QUESTIONS
- What toxicity and dosing concerns are there with elacestrant?
- What are your reactions to the safety data from the phase 3 EMERALD study (NCT03778931)?
- How do they compare with your experience with other standard therapies used in the second- and third-line setting (eg, everolimus plus exemestane or alpelisib [Piqray] plus fulvestrant)?
GRADISHAR: There was a comment [from Dr Narayan] that her patient [taking elacestrant] was very tired. This is one of the more common AEs that we see with it, as well as nausea.1 Nausea is a little [more common] than in other endocrine therapies. But the frequency of high-grade toxicity related to elacestrant is very uncommon. It stacks up quite well to other options. The fraction of patients who had grade 3 or 4 toxicity was similar…with an uptick of just a bit with elacestrant compared with other endocrine therapy. The fraction of patients who had to discontinue the drug was very [small, as was the number of] dose reductions [Table1].
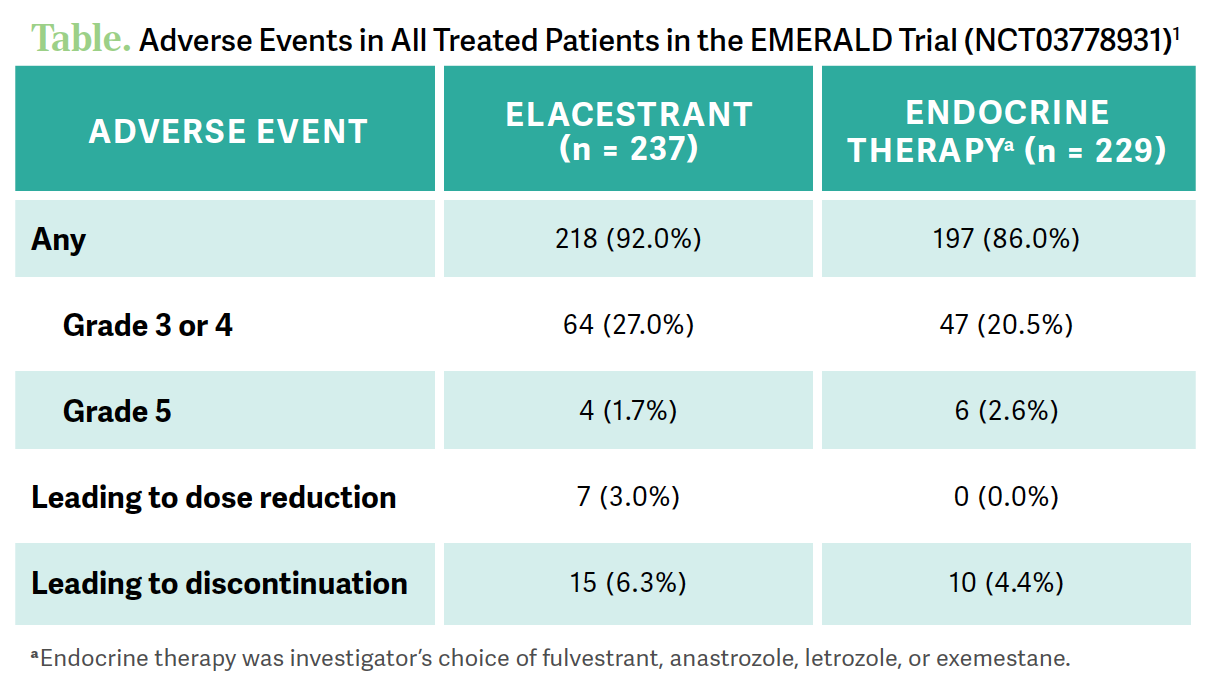
GRADISHAR: You can reduce the dose from the recommended starting dose [Figure2]. With the first dose reduction, you go down [from 345 mg] to 258 mg, second to 172 mg, and if they’re still having significant toxicity you would discontinue. But for grade 1 and 2 toxicity, you don’t necessarily have to change anything with the dose. It’s only in the unusual circumstances where patients have high-grade, meaning grade 3 or 4 AEs, that you have to not only interrupt but wait until the toxicity resolves and then restart at a lower dose.
GRADISHAR: [Do the EMERALD data1] in any way influence [how you treat] ESR-mutated patients, or those patients who may have both an ESR mutation and a [PI3K] mutation?
KHASAWNEH: Yes, in my practice, this is something important and practice-changing, because the biology is translating it into a better clinical outcome.

GRADISHAR: What has your experience been with alpelisib? That might be an option in those patients who have both mutations. You might choose one of these drugs over the other. What’s your experience to date with alpelisib?
KHASAWNEH: I have not used it…. We have a partner in our practice who sees most of the [newer patients with] breast cancer.
ANWAR: One patient I had with [the ESR mutation] had the PI3K mutation too, but I chose elacestrant because of its better toxicity profile.
GRADISHAR: Some of you have used [elacestrant] a couple of times, some not at all. Hearing about the toxicity, does it give you any sense compared with other combinations you might use? Alpelisib does have issues with hyperglycemia and rashes.3 And with everolimus, patients can develop some of the same problems, [such as] mucositis.4 Is there anything about elacestrant that makes you worried compared with the other options that may be available?
NARAYAN: I think from an AE profile [perspective], elacestrant would probably be a lot better tolerated. I used alpelisib 3 years ago in a patient and they had all kinds of problems [such as]…low phosphate levels and hyperglycemia. We had to dose reduce her a couple of times, and it’s just not the best experience. I would prefer elacestrant’s [toxicities].
SIDDIQUI: A couple of times I had to stop [a patient’s alpelisib because of] toxicities: Once for diarrhea and the other time she thought she took 2 pills instead of 1 and was admitted [to the hospital] with a blood sugar of 600 mg/dL.
GRADISHAR: So nobody is necessarily put off by the toxicity profile of elacestrant at this point. For breast tumors that are ER positive and HER2 negative, there was at least somebody who was more inclined to give fulvestrant [rather] than elacestrant, fulvestrant being an intramuscular shot and elacestrant being a pill. Fulvestrant has a good toxicity profile; elacestrant has a few potential AEs. [Investigators in the] EMERALD trial culled the patients who received fulvestrant [as a comparator], and elacestrant-treated patients did better.1
SIDDIQUI: What about continuing the CDK4/6 inhibitor and switching from an AI to fulvestrant?
GRADISHAR: Though you could do it, the data we have with sequential CDK4/6 inhibitors are limited, as you might expect.
REFERENCES
1. Bidard FC, Kaklamani VG, Neven P, et al. Elacestrant (oral selective estrogen receptor degrader) versus standard endocrine therapy for estrogen receptor-positive, human epidermal growth factor receptor 2-negative advanced breast cancer: results from the randomized phase III EMERALD trial. J Clin Oncol. 2022;40(28):3246-3256. doi:10.1200/ JCO.22.00338
2. Orserdu. Prescribing information. Stemline Therapeutics, Inc; 2023. Accessed October 3, 2023. https://tinyurl.com/4cy8yj5r
3. Piqray. Prescribing information. Novartis Pharmaceuticals; 2019. Accessed October 5, 2023. https://tinyurl.com/3z3kjuat
4. Afinitor. Prescribing information. Novartis Pharmaceuticals; 2020. Accessed October 5, 2023. https://tinyurl.com/ycxw4st4
