Martincic Reviews Risk, Diagnosis, and Management of Tumor Lysis Syndrome
During a Targeted Oncology™ Case-Based Roundtable™ event, Danko Martincic, MD, outlined the definition, risk factors, and treatment for patients with tumor lysis syndrome.
Danko Martincic, MD
Medical Oncologist
Beacon Cancer Care
Coeur d’Alene, ID

Targeted Oncology: How is tumor lysis syndrome (TLS) defined?
MARTINCIC: TLS is simply a situation where whatever malignancy we treat responds too well to treatment, and all of a sudden, the malignant cells fall apart. Most commonly, we are talking about hematologic malignancies. But there are cases of fast-growing malignancies, such as small cell lung cancer, that can lyse with the appropriate treatment.
The hallmark of TLS is that those cells fall apart, and the content of the cells either leaks into the circulation or undergoes catabolism and creates end products. So when we talk about TLS, for most [physicians] the synonym for it is hyperuricemia. Once it releases nucleic acid from the cells that are falling apart, purine bases undergo catabolism and then create high levels of uric acid. Uric acid goes to the kidneys, crystalizes there, and creates acute renal failure.1,2
The side product of purine catabolism is elevated phosphates, so hyperphosphatemia is going to be the next adverse event. [And] when these cells fall apart, a lot of potassium is going to be released from them, and hence, there is hyperkalemia.1,2
To summarize, hyperuricemia, hyperphosphatemia, hyperkalemia, [and also] hypercalcemia and uremia are indicators for TLS.1,2
Beyond acute hyperuricemia and acute renal failure, of which metabolic derangements are you particularly mindful?
Hyperkalemia leads to cardiac arrhythmias and sudden death. That is something that we don’t want to see. Both hyperkalemia and hyperuricemia are causes for concern for acute renal failure and associated consequences. We’re going to be worried about both of these things because they can result in an immediate, unwanted outcome.
What are the most important factors to consider when evaluating TLS risk?
TLS requires us to take into consideration a couple of different things. Primarily, [we need to consider] the type of disease that we are treating. Burkitt lymphoma, acute lymphoblastic leukemia [ALL], and diffuse large B-cell lymphoma [DLBCL] with an extremely high Ki67 score are classical diseases that may respond to treatment very fast and efficiently and create TLS.3
[Some] diseases have a low risk of TLS. [There is] a less than 1% chance that it’s going to happen.3 [The members of this category of diseases] have changed, simply because we have new treatment options that are quite efficient and effective and may lead to TLS even in diseases that were classically considered to be of intermediate or low risk of TLS. There is the classic story about chronic lymphocytic leukemia [CLL] being intermediate risk, with about a 3% chance of associated TLS, and then it became high risk when we developed venetoclax [Venclexta].
The primary risk factor is [the disease that is being treated—for example,] diseases with rapid cellular proliferation. We’ve seen patients with DLBCL, where the extent of the lymphadenopathy [and] stage IV involvement of the bone marrow is such that the number of cells in circulation and in the lymph nodes is so high that it creates a very fertile ground for TLS. We also think about the sensitivity of these particular diseases to the treatments that we use.3,4
What other factors contribute to TLS risk?
Among [factors] that commonly complicate the situation are involvement of the kidneys and the kidneys losing the capability to be as effective as they can be, in terms of managing all the electrolyte abnormalities. Bone marrow involvement is always a high risk, and splenomegaly creates a pool of cells that is not visible but present.3,4
Patient-related factors play a major role. I always worry about preexisting renal disease, and I certainly worry about congestive heart failure. I’m always worried about patients who have a disease that is at high risk for TLS and who also have a high tumor burden and congestive heart failure.3,4 The amount of fluid that we can give these patients is obviously limited, [given the] heart function. Older age brings its own set of limits3,4 because the organs do not function the way they used to.
Hypovolemia and hypotension are risk factors.3,4 However, since we know what we’re dealing with, I think there’s a good chance for us to take care of all these problems even before we start treatment.
Last, but not least, are treatment-related factors, [including the] intensity of cytoreductive therapy.3,4 For the most part in high-risk TLS disease, we use a combination of chemotherapies. We don’t use single-agent treatment to treat Burkitt lymphoma or high– proliferation rate DLBCL. [Another treatment-related risk factor] is [a lack] of adequate hydration during cytoreductive therapy. And finally, patients being on some kind of medication that may be nephrotoxic [is another risk factor].3,4
With the invention of new agents and their efficacy in treatment of some of these malignancies, we have created a situation where treatment itself increases the chance of TLS.
Which diseases pose the highest risk for TLS?
The usual suspects include Burkitt leukemia/lymphoma, with or without elevated lactate dehydrogenase levels, and ALL with a white blood cell count of at least 100,000/μL. I’ve been dealing with acute myeloid leukemia for a long time, especially in patients who present with a high white blood cell count [at least 50,000/μL, up to 100,000/μL]. Even though they are prone to developing TLS, my experience is that the risk is less than that of ALL and Burkitt lymphoma. Lymphoblastic lymphoma, T-cell leukemia/lymphoma, and DLBCL [are other high-risk diseases]. There are forms of mantle cell lymphoma that are extremely aggressive and bulky and that may create TLS. With CLL, if you have a bulky lymphadenopathy, splenomegaly, and an extremely high lymphocyte count, that is something that we should consider [high risk], especially in light of venetoclax treatment.5
Which treatments pose the greatest risk for TLS or uric acid elevation? Do such treatments represent newcomers to the landscape?
Such agents are not new to us, but they are outside of the classical chemotherapy regimens [cyclophosphamide (Cytoxan), doxorubicin hydrochloride, vincristine, high-dose cyclophosphamide, (cytarabine; Ara-C), and high-dose methotrexate (Trexall)]. Obviously, [the new treatments] have given us a good chance to be effective in the treatment of appropriate diseases, but they raised a question about whether diseases that historically were not considered to be prone to TLS [would] become such.
For example, we all use carfilzomib [Kyprolis], and we love it because of its efficacy. [However,] it creates a situation where, in multiple myeloma, we slightly increase the chance of TLS.6 TLS in multiple myeloma is extremely rare. A study from Arkansas, published [in 1999], had 1400 patients, and 2 of them developed TLS.6 However, carfilzomib and other agents increase the chance of [patients with multiple myeloma developing] TLS.7
I don’t have any experience with TLS caused by lenalidomide [Revlimid], but apparently it is [a risk].8 I like to use obinutuzumab [Gazyva] as an introductory debulking treatment in a high-risk patient with CLL whom I’d like to treat with venetoclax. But, apparently, obinutuzumab may cause TLS, too.9
What are important diagnostic considerations for TLS?
A couple of things are very important, in terms of setting the stage for the treatment of these patients. [When discussing] the Cairo-Bishop definition of TLS, I usually stick to the part that is probably less well known or [considered]: a 25% increase in hyperkalemia [and] hyperphosphatemia, a 35% decrease in hypercalcemia, and a 25% increase in hyperuricemia. With a 25% increase, we are already in TLS. That’s one thing that I’d like to keep in mind, and when we talk about the Cairo-Bishop definition, I am staying [on the topic] of the laboratory [definition of] TLS, not the clinical [definition of] TLS.10
Why not clinical TLS? Because clinical TLS includes sudden death,10 which is too little, too late. In my mind, the most important part of the Cairo-Bishop criteria is laboratory[-defined] TLS: increased levels of uric acid, potassium, and phosphorous above the high normal levels and a decreased calcium level below the low normal level. Laboratory TLS [can also be defined as a] 25% change from the baseline [of any of these parameters].10 [Such a change] tells us that things are moving in the wrong direction. I don’t like to find myself in a situation of cardiac arrhythmia or seizures, and definitely not sudden death. We would like to do everything to prevent clinically defined TLS.
How does TLS manifest clinically?
This is something that I like to avoid, but it’s good to know…. It’s good to have a feeling for how things are happening 12 to 72 hours [after the initiation of cytotoxic chemotherapy]. Clinical symptoms of TLS include nausea, vomiting, diarrhea, anorexia, weakness, and lethargy. Lethargy makes me worried. [I] definitely [am concerned with] cardiac dysrhythmia or seizures. Those are the things that I’m desperately trying to avoid, and I’m sure that’s [not news to anyone]. A long time ago, we were hydrating patients and putting them on allopurinol [Zyloprim], but we still had a higher percentage of patients developing TLS and [related] problems than [we do now,] since we discovered and started to use rasburicase [Elitek].2
What guidelines can help physicians prevent TLS?
In the low-risk patients, we watch and wait, do intravenous [IV] fluids, and maybe put patients on allopurinol. In the low-risk patients, it’s [an individualized decision whether to] put a patient on full-blown IV fluids and allopurinol just to be safe, or just [to wait and] see what’s going to happen, given the very low risk of TLS.3,5
Intermediate risk certainly raises more questions about what we need to do, and most of us will reach for IV hydration unless it is not permitted by the patient’s condition. Prophylactic allopurinol continues to be a valuable measure. Allopurinol primarily works by reducing the production of uric acid; it does not influence the other parts of TLS. If a patient already has high uric acid levels, allopurinol is not going to be useful. Allopurinol prevents the formation of new uric acid. Part of the problem that we might have is [providing] very vigilant laboratory monitoring and hydration of patients. That’s why we reach out to hospital admission.3,5
Rasburicase is considered as an initial antihyperuricemic agent in patients with intermediate risk.3,5 Again, I think it’s going to be [an individualized] choice. I have to admit that I’m a little [cautious], and I like to use rasburicase if I see evidence of any of the Cairo-Bishop laboratory criteria for TLS. One admission to the hospital for TLS is way more expensive than 1 injection of rasburicase.
For high-risk patients, [the recommendations are] aggressive IV hydration, prophylactic rasburicase, vigilant monitoring of laboratory test results, and allopurinol. [The fact that allopurinol should be substituted for rasburicase in] patients with glucose- 6-phosphate dehydrogenase deficiency was something that I didn’t know. I have never had such a case and have never thought about that, but it is interesting.3,5
How does hydration play a role in the prevention of TLS?
For low-risk patients, aggressive oral hydration is recommended. For high-risk patients, IV hydration is recommended. We should take patient characteristics into consideration.11 I don’t like to see congestive heart failure [in a] patient I need to treat for Burkitt lymphoma.
We try to do IV hydration as much as we can. I usually like to start these treatments early in the morning so I can keep the patient in the chemotherapy room from 8:00 am to 5:00 pm and give them enough fluids that I feel comfortable for them to go home. There are cases where we admit the patient to the hospital just to make sure that things are not going to get worse.
Has there been a study to compare the efficacy of rasburicase plus allopurinol vs the efficacy of either single agent in the control of plasma uric acid?
There was a phase 3 randomized trial [NCT00230178] in patients [at risk for] TLS. This trial had 3 arms: single-agent rasburicase, rasburicase in combination with allopurinol, and single-agent allopurinol.12 [Single-agent] rasburicase was given on day 1 [through] day 5, which, I would guess, most of us are not doing, for a couple of good reasons. However, this was a study, and I suppose [the rasburicase treatment] had to match the allopurinol treatment in order for the study to yield comparable data.
[The results of the study] give us a degree of confidence that rasburicase has a greater effect on plasma uric acid [than does allopurinol], with response rates of 87% for rasburicase [P = .001 vs allopurinol], 78% for the combination [P = .06 vs allopurinol], and 66% for allopurinol [Figure12]. I think the combination of rasburicase and allopurinol is probably what most of us are doing.
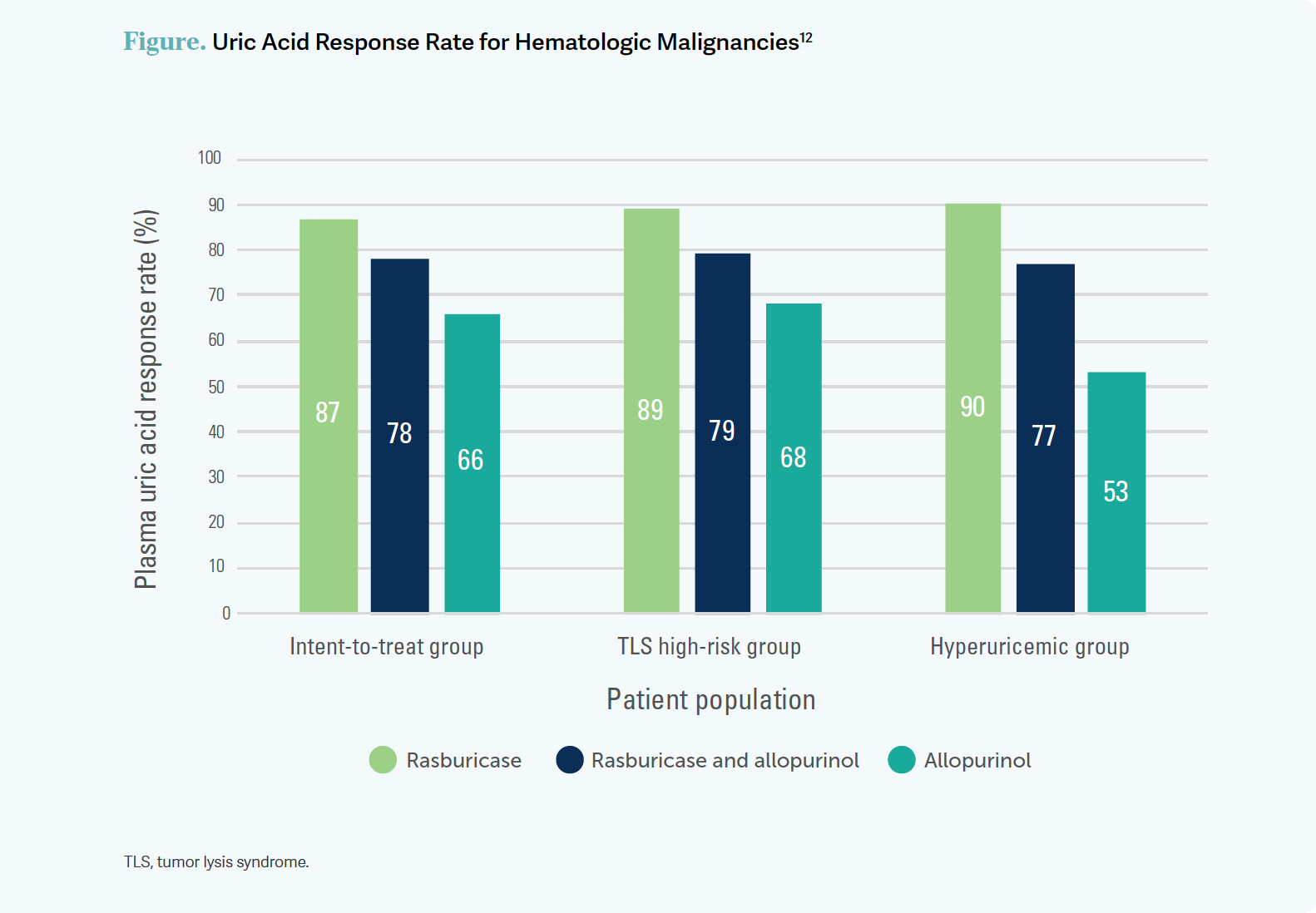
Allopurinol does not degrade uric acid that is already made. It decreases the [production of ] new uric acid. Therefore, in the single-agent allopurinol arm, the level of uric acid decreased slowly. On the other hand, 1 [dose] of rasburicase within [4] hours [brought] uric acid down to extremely low levels. The combination of rasburicase and allopurinol [produced uric acid levels that were] closer to those of the rasburicase arm [than to those of the allopurinol arm]. [It was] very effective. [Three] treatments exerted the same effect as did 5 treatments of rasburicase in this study.12
The incidence of TLS and renal events was compared among the treatment options. In the rasburicase arm, there were 21 laboratory TLS events vs 41 events in the allopurinol arm [P < .05 for rasburicase vs allopurinol] and 27 events in the combination arm. There were different levels of other abnormalities. Hyperuricemia affected 8 patients in the rasburicase arm vs 29 in the allopurinol arm and 12 in the combination arms. The results for hyperphosphatemia were slightly less impressive. The results for hypercalcemia and, especially, hyperkalemia were similar. Apparently, allopurinol gives a little advantage in terms of hyperkalemia. One has to keep that in mind.12
The incidence of [elevated] creatinine was slightly higher in the allopurinol arm [10 patients] than in the rasburicase arm [8 patients]. Interestingly, the incidence in the combination arm was the same as in the allopurinol arm [10 patients]. The incidence of renal failure was interesting; renal failure happened in 4 patients in the rasburicase arm, in 2 patients in the allopurinol arm, and in 9 patients in the combination arm.12 Other factors of TLS besides uric acid production contribute to renal failure.
There were not too many drug-related adverse events associated with rasburicase [4 events out of 92 patients], and not too many with allopurinol [5 events out of 91 patients],12 so both of these agents were extremely well tolerated.
REFERENCES
1. Pession A, Melchionda F, Castellini C. Pitfalls, prevention, and treatment of hyperuricemia during tumor lysis syndrome in the era of rasburicase (recombinant urate oxidase). Biologics. 2008;2(1):129-141. doi:10.2147/btt.s1522
2. Coiffier B, Altman A, Pui CH, Younes A, Cairo MS. Guidelines for the management of pediatric and adult tumor lysis syndrome: an evidence-based review. J Clin Oncol. 2008;26(16):2767-2778. doi:10.1200/JCO.2007.15.0177
3. Williams SM, Killeen AA. Tumor lysis syndrome. Arch Pathol Lab Med. 2019;143(3):386-393. doi:10.5858/arpa.2017-0278-RS
4. Matuszkiewicz-Rowinska J, Malyszko J. Prevention and treatment of tumor lysis syndrome in the era of onco-nephrology progress. Kidney Blood Press Res. 2020;45(5):645-660. doi:10.1159/000509934
5. Cairo MS, Coiffier B, Reiter A, Younes A; TLS Expert Panel. Recommendations for the evaluation of risk and prophylaxis of tumour lysis syndrome (TLS) in adults and children with malignant diseases: an expert TLS panel consensus. Br J Haematol. 2010;149(4):578-586. doi:10.1111/j.1365-2141.2010.08143.x
6. Fassas AB, Desikan KR, Siegel D, et al. Tumour lysis syndrome complicating high-dose treatment in patients with multiple myeloma. Br J Haematol. 1999;105(4):938-941. doi:10.1046/j.1365-2141.1999.01467.x
7. Kyprolis. Prescribing information. Amgen; 2022. Accessed April 15, 2023. https://bit.ly/43STcJG
8. Revlimid. Prescribing information. Bristol-Myers Squibb; 2023. Accessed April 15, 2023. https://bit.ly/41vYCZs
9. Gazyva. Prescribing information. Genentech; 2022. Accessed April 15, 2023. https://bit.ly/3oBFKJU
10. Puri I, Sharma D, Gunturu KS, Ahmed AA. Diagnosis and management of tumor lysis syndrome. J Community Hosp Intern Med Perspect. 2020;10(3):269-272. doi:10.1080/20009666.2020.1761185
11. Goodrich A. Advanced practice perspectives on preventing and managing tumor lysis syndrome and neutropenia in chronic lymphocytic leukemia. J Adv Pract Oncol. 2021;12(1):59-70. doi:10.6004/jadpro.2021.12.1.5
12. Cortes J, Moore JO, Maziarz RT, et al. Control of plasma uric acid in adults at risk for tumor lysis syndrome: efficacy and safety of rasburicase alone and rasburicase followed by allopurinol compared with allopurinol alone--results of a multicenter phase III study. J Clin Oncol. 2010;28(27):4207-4213. doi:10.1200/JCO.2009.26.8896
