Treating Rare Driver Mutations in NSCLC: EGFR Exon 20 Insertions
During a Case-Based Roundtable® event, Misako Nagasaka, MD, discussed treatment for a patient with non–small cell lung cancer and an EGFR exon 20 insertion.
Misako Nagasaka, MD, PhD
Associate Clinical Professor
Division of Hematology/Oncology, Medicine
School of Medicine
University of California, Irvine
Orange, CA

CASE SUMMARY
- An Asian woman aged 64 years presented with persistent dry cough and mild progressive dyspnea. During the past 4 months, she had an unintentional weight loss of 8 lb.
Medical and Social History
- Hypertension, controlled with metoprolol at 100 mg daily
- Hyperlipidemia, treated with atorvastatin 20 mg daily
- Mother of 2 adolescents
- Smoking history: never smoker
Focused Physical Examination
- Right lower lobe auscultation revealed decreased breath sounds.
- ECOG performance status: 1
- Forced expiratory volume in 1 second (FEV1): 78%; postbronchodilator FEV1/forced vital capacity: less than 0.74; diffusing capacity for carbon monoxide: 69%
Imaging Studies
- CT chest scan: 3.5-cm mass in right lower lobe, small right pleural effusion with nodularity; multiple mediastinal lymph nodes
- PET/CT scan: increased uptake along lumbosacral vertebrae and left scapula
- Brain MRI negative for metastases
Bronchoscopy With Biopsy and Mediastinal Node Endobronchial Ultrasound
- Identified suspicious mass for non–small cell lung cancer (NSCLC) plus 3 mediastinal lymph nodes measuring 2.25 cm, 2.1 cm, and 1.8 cm in diameter
- TTF1-positive adenocarcinoma
- Stage IVB
Molecular Profile
- Negative PD-L1 expression
- Tissue next-generation sequencing (NGS) showed EGFR exon 20 insertion mutation (V769_D770insASV).
PEERS & PERSPECTIVES IN ONCOLOGY®: Please discuss the EGFR oncogenic driver mutations as seen in this patient’s molecular profile.
NAGASAKA: In the EGFR-mutated population, exon 19 and exon 21 mutations are the most common.1,2 The third most common is the EGFR exon 20 group. It is important to note that EGFR tyrosine kinase inhibitors [TKIs] used for common EGFR mutations are not effective in EGFR exon 20 insertion mutations for the most part. It is also important to note that EGFR exon 20 insertions are very heterogeneous with numerous variants where polymerase chain reaction testing can miss up to 50% of these, and it’s another reason to consider NGS upfront.
Some of the characteristics of EGFR exon 20 insertions are similar to the general or common EGFR mutations, which include seeing more in women, non-smokers, in the Asian population, and in those with adenocarcinoma histology. But what’s important is that it is different, in a sense; the usual first-, second-, third-generation EGFR TKIs like gefitinib [Iressa]…osimertinib [Tagrisso], or immunotherapy don’t work for those with EGFR exon 20 insertion mutations. It’s also important not to confuse EGFR exon 20 insertion mutations with the EGFR exon 20 T790M mutation, which is a very common resistance mutation that we used to see more often in the past when we were using first- or second-generation EGFR TKIs upfront.
How do patients with EGFR exon 20 insertion mutations do on therapy in real-world settings?
Retrospective studies using the Flatiron database have shown significant differences in the real-world overall survival [OS], real-world progression-free survival [PFS], and real-world time-to-new treatment.3 For example, the median real-world OS was 25.5 months in patients with common EGFR mutations, whereas it was only 16.2 months in EGFR exon 20 insertions.
There is another large data-based study using the Flatiron database and it is shocking to see that even through the data extraction years of 2011 to 2020, which I believe is fairly recent, 33.2% were not tested for EGFR mutations.4 Out of those who have testing for EGFR, only 304 patients or 4.9% of patients had EGFR exon 20 insertions. It is rare, but important to identify.
The treatment for EGFR exon 19 deletion or exon 21 mutation is straightforward, with osimertinib being the preferred first-line therapy.5 At the time of progression, local therapy and continuation of osimertinib could be considered when patients are asymptomatic and there’s limited progression. If there are multiple lesions, we typically consider switching to systemic therapy. Traditionally that has been chemotherapy in this setting. The treatment landscape is different with EGFR exon 20 insertion mutation and this continues to evolve. Currently, EGFR exon 20 insertion mutations, when they are diagnosed, should be treated with platinum-based chemotherapy up front and at the time of progression for subsequent therapy, amivantamab [Rybrevant] has been given FDA approval.
CASE UPDATE
Treatment
- The patient was started on chemotherapy doublet (cisplatin and pemetrexed).
- She experienced a good partial response to this regimen.
Four months after initiation of chemotherapy
- The patient reported worsening mid- to lower back pain and dyspnea on mild exertion.
- Repeat imaging studies CT scan showed disease progression.
- Repeat brain MRI showed no metastases.
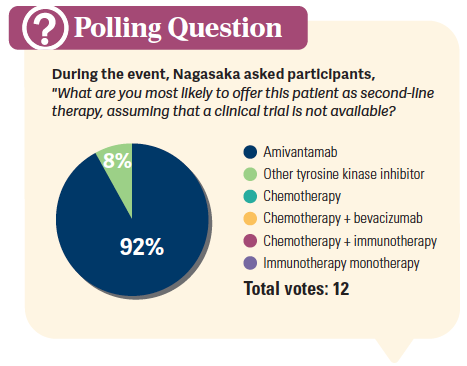
What is the background for using amivantamab in the EGFR exon 20–mutated NSCLC population?
Amivantamab is a fully humanized bispecific immunoglobulin G1 [antibody] and it targets EGFR and c-MET.6 c-MET is a known resistance pathway for EGFR, and by [targeting both], amivantamab has the potential to provide clinical benefit in EGFR-driven NSCLC, including the TKI-resistant population and exon 20 insertion mutation population. The interesting thing about amivantamab is that it’s not just an antibody; it appears to activate the immune system and has immune cell–directing activity of trogocytosis and receptor degradation, in addition to inhibition of ligand binding. Trogocytosis is where amivantamab causes injury to the tumor cell membrane and activates the macrophages to eat the tumor cells, which leads to cancer cell death. Amivantamab has demonstrated monotherapy activity in patients with diverse EGFR mutations, including the common mutations as well as T790M, C797S, exon 20 insertions, and c-MET.
Which data led to the approval of this drug in patients with NSCLC as second-line therapy?
CHRYSALIS [NCT02609776] was the phase 1 study that led to the approval of amivantamab in the second-line setting of EGFR exon 20 insertions, which was cohort D.7 CHRYSALIS itself was a multicohort study and they had different cohorts, so they accrued patients with different settings. We have data with post-EGFR third-generation TKI, C797S, [and] EGFR exon 20 insertions, which led to the approval of amivantamab, and we have more data on MET.
These patients were primarily treated already with chemotherapy platinum doublet agent. In that setting, the overall response rate was 40% in a blinded independent review and 36% by investigator review. The clinical benefit rate was 74%. The median PFS was 8.3 months and median OS was 22.8 months.
What other data have come out for amivantamab?
The phase 3 PAPILLON [NCT04538664] results were some of the most exciting data presented at the European Society for Medical Oncology Congress 2023.8 This was a first-line study for patients with EGFR exon 20 insertions. The question this study is trying to answer is if we combine amivantamab and chemotherapy up front and compare that with the current standard of care—chemotherapy—how would the results be? Are there going to be great results? Is there an advantage of giving amivantamab early on? And there was.
The distributions of age and sex were consistent with what we see in the real world.9 The study was positive for its primary end point, which was PFS by independent review, and the HR was 0.40.8 That is pretty impressive, with a median PFS of 11.4 months with the chemotherapy plus amivantamab arm and 6.7 months for the chemotherapy alone arm. The objective response rate was also superior with amivantamab plus chemotherapy at 73% vs only chemotherapy at 47%. The duration of response was also longer with chemotherapy plus amivantamab. The median OS was not estimable for amivantamab plus chemotherapy and 24.4 months for chemotherapy alone, which provided an HR of 0.67. There is an unmet need for EGFR exon 20 insertions, and this first-line combination is looking really promising and it has a chance of getting approved.
The overall safety profile was consistent with chemotherapy-related adverse events [AEs], as one would imagine, like neutropenia, anemia, and with EGFR- and c-MET-related toxicities in the chemotherapy plus amivantamab arm.
What are some practical considerations of amivantamab for patients with NSCLC and exon 20 insertions?
For those of us who haven’t used amivantamab, it is an interesting drug, but it has its quirks, too. The mechanism of action is a bispecific EGFR and MET receptor–directed antibody. The administration is a little bit complicated, especially on the first day because 66% of patients were seen to have infusion-related reactions.7,10 You want to premedicate them with medications like steroids and Benadryl [diphenhydramine]. It does take some time. For those of us who have used amivantamab, it almost feels like the body just needs to get a little bit of exposure to this drug so that it gets used to it. Then from day 2, patients don’t react to it anymore.
The way that this drug is typically given is weekly dosing for the first 4 weeks, followed by every other week starting from week 5. But for the first week, day 1 is split between day 1 and day 2, because most people will have an infusion-related reaction. That means we’re going to be stopping the infusion, slowing down that infusion, so it takes forever to infuse on day 1. You can’t complete the infusion on day 1, so you usually want to split the dose in day 1 and day 2. For whatever reason on the second day, most patients do not have an infusion-related reaction.
The more long-term AEs that I see using this drug are rash and paronychia, which is part of the EGFR inhibition pathway–related AEs. Also, since we’re inhibiting MET, you can see some lower extremity edema. Those are things to consider and think about. There is a subcutaneous formulation of amivantamab being investigated in clinical trials and I think that would make it much easier for patients because it would be a quick injection.
REFERENCES:
1. Byeon S, Kim Y, Lim SW, et al. Clinical outcomes of EGFR exon 20 insertion mutations in advanced non-small cell lung cancer in Korea. Cancer Res Treat. 2019;51(2):623-631. doi:10.4143/crt.2018.151
2. Meador CB, Sequist LV, Piotrowska Z. Targeting EGFR exon 20 insertions in non–small cell lung cancer: recent advances and clinical updates. Cancer Discov. 2021;11(9):2145-2157. doi:10.1158/2159-8290.CD-21-0226
3. Bazhenova L, Minchom A, Viteri S, et al. Comparative clinical outcomes for patients with advanced NSCLC harboring EGFR exon 20 insertion mutations and common EGFR mutations. Lung Cancer. 2021;162:154-161. doi:10.1016/j.lungcan.2021.10.020
4. Lin HM, Yin Y, Crossland V, Wu Y, Ou SI. EGFR testing patterns and detection of EGFR exon 20 insertions in the United States. JTO Clin Res Rep. 2022;3(3):100285. doi:10.1016/j.jtocrr.2022.100285
5. NCCN. Clinical Practice Guidelines in Oncology. Non-small cell lung cancer; version 3.2024. Accessed March 27, 2024. https://www.nccn.org/guidelines/guidelines-detail?category=1&id=1450
6. Vyse S, Huang PH. Amivantamab for the treatment of EGFR exon 20 insertion mutant non-small cell lung cancer. Expert Rev Anticancer Ther. 2022;22(1):3-16. doi:10.1080/14737140.2022.2016397
7. Park K, Haura EB, Leighl NB, et al. Amivantamab in EGFR exon 20 insertion–mutated non–small-cell lung cancer progressing on platinum chemotherapy: initial results from the CHRYSALIS phase I study. J Clin Oncol. 2021;39(30):3391-3402. doi:10.1200/JCO.21.00662
8. Girard N, Park K, Tang K, et al. LBA5 Amivantamab plus chemotherapy vs chemotherapy as first-line treatment in EGFR exon 20 insertion-mutated advanced non-small cell lung cancer (NSCLC): primary results from PAPILLON, a randomized phase III global study. Ann Oncol. 2023;34(suppl 2):S1304. doi:10.1016/j.annonc.2023.10.060
9. Zhou C, Tang KJ, Cho BC, et al. Amivantamab plus chemotherapy in NSCLC with EGFR exon 20 insertions. N Engl J Med. 2023;389(22):2039-2051. doi:10.1056/NEJMoa2306441
10. Rybrevant (amivantamab). Prescribing information. Janssen Biotech; 2021. Accessed March 28, 2024. www.accessdata.fda.gov/drugsatfda_docs/label/2021/761210s000lbl.pdf

Overcoming Barriers in Cancer Clinical Trials: A Path Forward for Better Patient Care
April 29th 2024Clinical trials play a pivotal role in developing effective therapies, yet their integration is challenged by issues such as insufficient reimbursement structures, misaligned incentives, physician burnout, and a complex regulatory environment.
Read More
Gholam Analyzes Treatment Outcomes for Advanced HCC in Child-Pugh B Population
April 28th 2024During a live Community Case Forum event in partnership with the Tennessee Oncology Practice Society, Pierre Gholam, MD, examined the current state of treatment for patients with hepatocellular carcinoma, looking in particular at what data is available for those with Child-Pugh B and C status who have poorer outcomes and have limited data from prospective clinical trials.
Read More