Lifileucel Approval Signals a Turning Point in Melanoma Therapy
Lifileucel's FDA approval marks a breakthrough in melanoma treatment, offering hope with promising antitumor activity. While its potential extends to various solid tumors, challenges persist.
Howard D. Edington, MD

The recent approval of the cellular therapy lifileucel (LN-44; Amtagvi) marks a significant advance in the treatment of patients with melanoma, especially those with advanced stages of the disease, as it offers a novel personalized approach.1
According to Howard D. Edington, MD, the foundation of lifileucel lies in the body's innate ability to eliminate cancerous cells.
"With [my] experience at the National Cancer Institute…we were doing research into the immune manipulations and [whether] we could harness the immune system to cure specifically melanoma, because that was one of the cancers that had early on demonstrated the immune component of the patient to the cancer," explained Edington, director of the Melanoma and Skin Cancer Center at Allegheny Health Network, in an interview with Targeted OncologyTM.
"The observation that some primary melanomas disappear completely in patients was one that had been known for many years, but it clearly suggested that patients were able to get rid of their own cancer," Edington continued.
Lifileucel is a tumor infiltrating lymphocyte (TIL) therapy for patients with advanced melanoma who progressed on at least 1 systemic therapy, including a PD-1 inhibitor, and if BRAF V600 positive, a BRAF inhibitor with or without a MEK inhibitor. The autologous TIL cell therapy has demonstrated clinically meaningful antitumor activity in patients with advanced melanoma.2
“The way it works is we harvest a tumor from a patient, and then we obtain those T cells that are there in the tumor. We think that the majority of those T cells are tumor-directed, and they recognize multiple tumor neoantigens, so we take them out," explained Omid Hamid, MD, in an interview with Targeted OncologyTM.
Omid Hamid, MD

The treatment process involves shipping the harvested tumor to a central facility where T cells are expanded to billions before being returned to the patient after lymphodepletion therapy.
“Lymphodepletion means chemotherapy to deplete the lymphocytes in the blood, those other T cells that are tumor nonspecific,” noted Hamid, who is chief of translational research and immunotherapy and director of melanoma therapeutics at The Angeles Clinic and Research Institute in Los Angeles, California.
Lifileucel has produced early and durable responses in patients with advanced melanoma previously treated with immune checkpoint inhibition.
“Lifileucel [treatment] involves patients who have stage IV disease, generally, so it is advanced melanoma, which fortunately is not the bulk of the patients that have melanoma. Most of them we can take care of. These are patients that have failed other treatments," Edington said.
One of the distinguishing features of lifileucel is its efficacy as a second-line therapy. The agent offers hope to patients who have not responded to or progressed on conventional treatments. According to Hamid, "…this is a second-line therapy that has shown, not only a significant objective response rate—this is 1 of the highest if not the highest in the second line—but it has also shown durability in the lifileucel therapy trial."
Behind the FDA Approval of Lifileucel
In February 2024, the FDA granted an accelerated approval to lifileucel for the treatment of patients with advanced melanoma who have progressed after receiving an anti-PD-1 antibody and, if BRAF V600 mutation positive, a BRAF inhibitor with or without a MEK inhibitor.1,2
The FDA primarily based its decision on findings from an efficacy population of 73 patients enrolled in cohort 4 of the phase 2 C-144-01 trial (NCT02360579) who were treated with lifileucel at the recommended dose. The objective response rate (ORR) in these patients was 31.5%, comprised of 3 (4.1%) complete responses (CRs) and 20 (27.4%) partial responses (PRs). Among patient responders, 56.5% of patients maintained their response at 6 months, 47.8% at 9 months, and 43.5% at 12 months.
Data supporting the use of lifileucel in this patient population also come from a pooled efficacy set from the C-144-01 trial consisting of 153 patients from cohorts 2 (n = 66) and 4 (n = 87). The ORR in this pool population was 31.4% (95% CI, 24.1%-39.4%).3 Long-term results for this population were shared at the 2023 ESMO Immuno-Oncology Congress.3
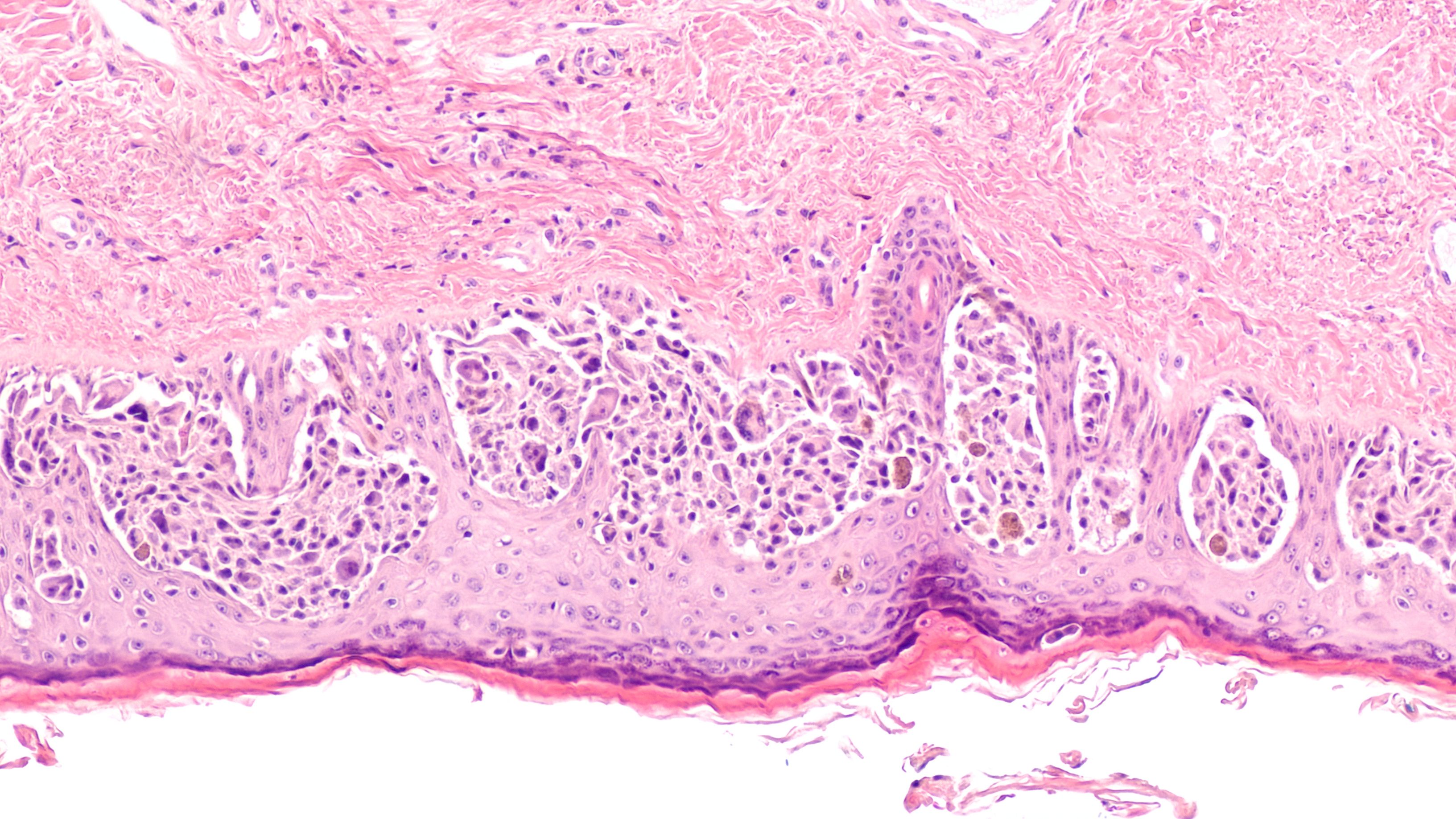
Tumor infiltrating lymphocytes in melanoma
"After 4 years of follow-up from the cohorts that received lifileucel... we have seen a response rate of 31.4%...the responses were not only rapid...but they were durable," explained Hamid.
Among the 153 patients, the median age was 56 years (range, 20-79). A total of 49.7% had positive PD-L1 expression (tumor proportion score ≥1%), lactate dehydrogenase (LDH) levels were at or below the upper limit of normal (ULN) in 45.8% of patients, and liver and/or brain lesions were seen in 47.1% of patients. The median sum of diameters for target lesions was 101.1 mm (range, 13.5-552.9), the percentage of patients who had 3 or more baseline lesions at anatomic sites was 71.2%, while 75.8% had more than 3 baseline target and nontarget lesions, and the median number of prior lines of therapy given to patients was 3 (range, 1-9).3
Further, 71.2% of patients experienced primary resistance to prior anti-PD-1/L1 therapy, and most patients who had advanced melanoma were heavily pretreated. Notably, 56.3% of responders to lifileucel had LDH levels at or below the ULN, and 62.5% had over 3 baseline target and nontarget lesions. The median sum of diameter for target lesions for responders was 68.8 mm (range, 13.5-552.9).
Eight patients had CRs and 40 patients had PRs. Additionally, 1.5 months was the median time from lifileucel infusion to best response (range, 1.3-30.4) and 6 patients improved from PR to CR as late as 2-plus years after treatment.
For all responders (n = 48), the median duration of response (DOR) was not reached (NR; 95% CI, 8.3-NR), and according to patterns of response, the median DOR was NR in early responders (n = 39; 95% CI, 6.1-NR). The median DOR was 19.8 months (95% CI, 4.1-NR) in the 9 late responders, 26.2 months (95% CI, 4.1-NR) in the 32 responders who did not have a deepened response, and NR (95% CI, 8.3-NR) in the 16 responders who had deepened responses.
“We have seen a paradigm with immunotherapy that you can begin with stability of disease and then get a deeper response or begin with a partial response and then after time get a more durable response. We have seen that when a response is being converted to complete response. In early responders, a median duration of response has not been reached yet, but we are seeing even those patients that respond later have durable responses,” added Hamid.
Looking at long-term survival, the median overall survival (OS) was 13.9 months (95% CI, 10.6-17.8) among all patients. The OS rate at 4 years was 47.3% (95% CI, 32.5%-60.7%), and according to patterns of responses, 4-year OS rates were 48.3% in early responders (95% CI, 31.9%-62.9%), 41.7% (95% CI, 10.9%-70.8%) in late responders, 37.2% (95% CI, 21.0%-53.5%) in responders without a deepened response, and 68.2% (95% CI, 39.5%-85.4) in responders with deepened responses.
Moreover, the safety findings observed with lifileucel in this study were consistent with the known profiles of nonmyeloablative lymphodepletion and interleukin-2 (IL-2). Notably, the incidence of treatment-emergent adverse effects (AEs) rapidly decreased within 2 weeks after lifileucel infusion. No new late-onset lifileucel-related serious AEs were reported.
“Lifileucel offers patients hope when just 10 to 15 years ago, there was not any hope. I think it is going to rewrite the statistics for patients having a whole variety of different types of cancer, which is obviously exciting for those of us that are in the business of cancer therapy,” said Edington.
Melanoma: © David A Litman - stock.adobe.com
TILVANCE-301
Since lifileucel was approved under the FDA’s accelerated approval program, continued approval of the cellular therapy may be contingent on further demonstration of its benefit in a confirmatory trial.
One such trial is the ongoing phase 3, multicenter, open-label TILVANCE-301 trial (NCT05727904), which is evaluating lifileucel plus pembrolizumab (Keytruda) compared with pembrolizumab alone in patients with untreated advanced melanoma. The estimated enrollment is 670 patients who will be randomized to 1 of 2 arms.4
“There is a TILVANCE trial, which is a phase 3, randomized trial between single-agent PD-1 vs PD-1 and TIL. That will give us a lot of information about the earlier roles of these therapies,” explained Hamid.
In arm A, patients are being treated with pembrolizumab, followed by the lifileucel regimen, which includes nonmyeloablative lymphodepletion, lifileucel infusion, and IL-2. In arm B, pembrolizumab monotherapy every 6 weeks until disease progression is being given to patients. Eligible patients in arm B will have the option to cross over to arm A.
Investigators are evaluating the coprimary end points of ORR and progression-free survival and the secondary end points of OS, CR rate, DOR, event-free survival, and incidence of AEs.
Enrollment in the trial is open to patients with histologically or pathologically confirmed stage IIIC, IIID, or IV unresectable metastatic melanoma who have an ECOG performance status of 0 or 1, a life expectancy of greater than 6 months, and adequate organ function.
Patients will be excluded from the study if their melanoma is of uveal or ocular origin, if they have symptomatic untreated brain metastases, if they have received prior therapy for metastatic disease, or if they have a BRAF V600 mutation-positive tumor that received prior immune checkpoint inhibitor therapy.
“It paves the way for us to continue to look towards more personalized therapies. What we do not have at this point is predictive markers, prognostic and predictive. We have this understanding that possibly those patients who have seen less PD-1 respond better, or those who have seen less lines of therapy may respond better, and those who have less volume of disease may respond better, but we still have a lot to learn in this field,” Hamid added.
Lifileucel’s Potential Across Multiple Tumor Types
Beyond melanoma, lifileucel paves the way for broader applications in solid tumors. Specifically, lifileucel may offer a novel approach to treating non-small cell lung cancer, head and neck cancer, cervical cancer, breast cancer, colorectal cancer, and sarcomas.
“There is a space for this and other cancers in the future,” explained Hamid. "The impact to the future of melanoma treatment is huge for lifileucel and other TIL therapies...these bring in this whole group of therapies as a viable developmental plan in melanoma and other solid tumors.”
The versatility of lifileucel also opens doors for exploration in a wide range of solid tumors, including pancreatic cancer, ovarian cancer, and renal cell carcinoma, among others. Research into the safety and efficacy of lifileucel across these indications is ongoing.
As with any new therapy, challenges persist. Some of the current obstacles being faced by clinicians involve familiarization with the treatment protocol and ensuring adequate support infrastructure. According to Hamid, collaboration and expertise in navigating these challenges are of the utmost importance.
"The initial push to have it as a therapeutic requires a multitude of people including oncologists, hospital administrators, pharmacists, etc," added Hamid.
Overall, the development of lifileucel showcases the encouraging impact of immunotherapy on cancer treatment. While research on the agent continues to unfold, lifileucel holds promise in the melanoma space, offering optimism to both patients and oncologists alike.