Allan Looks at Acalabrutinib Results as Treatment of Patients With CLL
John N. Allan, MD, discussed using acalabrutinib for the treatment of a 61-year-old woman with chronic lymphocytic leukemia.
John N. Allan, MD

John N. Allan, MD, assistant professor of Medicine, Division of Hematology and Medical Oncology at Weill Cornell Medicine, discussed using acalabrutinib for the treatment of a 61-year-old woman with chronic lymphocytic leukemia (CLL).
Targeted OncologyTM: What factors influence whether you recommend therapy initiation for this patient?
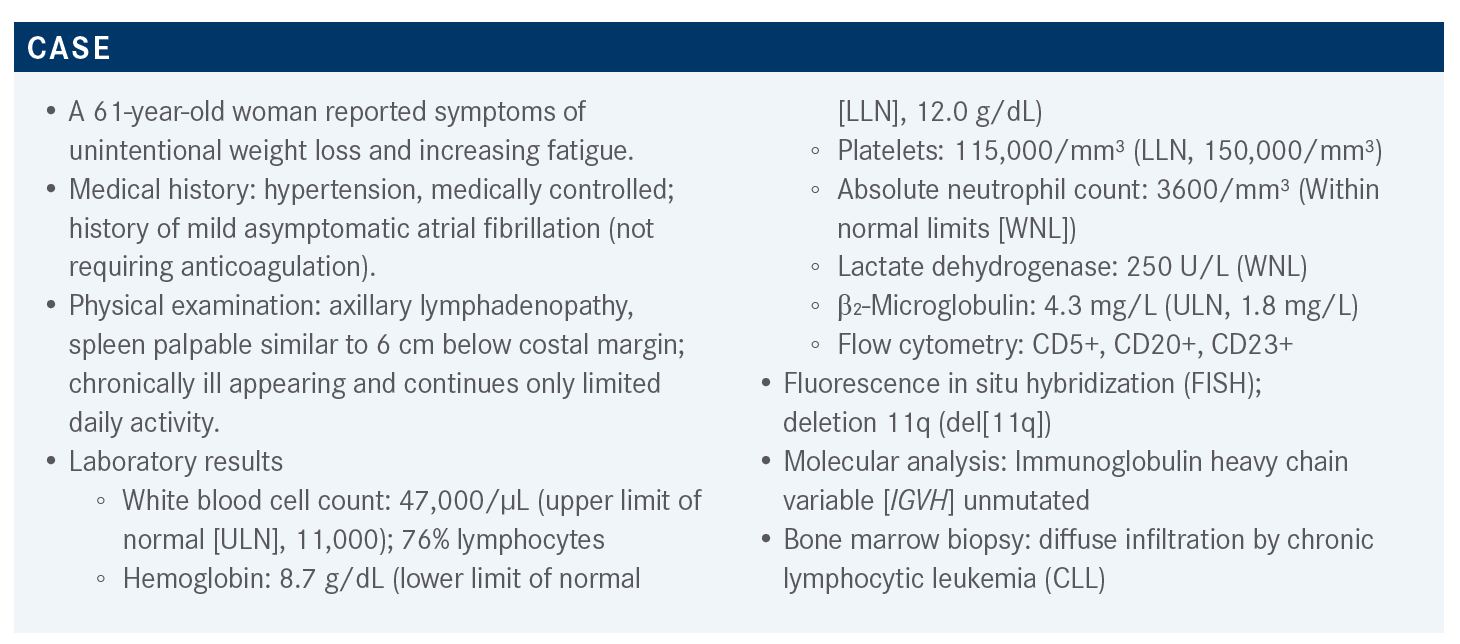
ALLAN: The patient is symptomatic and meets multiple indications to initiate treatment, according to the International Workshop on Chronic Lymphocytic Leukemia [iwCLL].1 She has progressive splenomegaly that is greater than 6 cm below the costal margin and down into the pelvis. She’s at stage III with hemoglobin less than 10 g/dL, which is an indication for therapy. The adenopathy, the B symptoms, the night sweats, and the fatigue are all factors and criteria to initiate treatment according to the iwCLL. So, there is no doubt this patient needs therapy.
How would you assess high, intermediate, or low risk for this patient and which tools do you use?
In terms of prognosis, she has some higher-risk features, namely unmutated [IGHV] and 11q deletion. Before we had targeted agents, these features were predictive of a high risk for poor responses to treatment, time to first treatment, etc. We do have an established and well-validated international prognostic index [IPI] for CLL that can be used to stratify patients into very high risk, high risk, intermediate, or low risk based on a point system that goes up to 10 points.2
Anything 4 points and above is considered high risk. So, 4 to 7 points are high and 8 to 10 points are very high risk. The factors considered in the CLL-IPI are age greater than 65 [1 point], [clinical stage (1 point)], elevated serum β₂-microglobulin [2 points], unmutated IGHV [2 points], and 17p deletion and/or TP53 mutation [4 points]. The CLL-IPI, which is a very useful tool for patient education, is very predictive for time to first treatment and for overall survival [OS] when using chemotherapy. It’s a little less clear how predictive it is in terms of OS when using targeted agents as frontline therapies. Therefore, these are the tools that can be used and the molecular tests that should be done.
In this case, it looks like all tests were done except for TP53 sequencing, which is recommended in the iwCLL guidelines because about 5% of patients who don’t have a 17p deletion may have a TP53 mutation and have the same prognosis.
Is it mandatory to test for CCND1 [formerly BCL1] translocation in this patient?
The CLL [FISH] panel has the break-apart probe for [chromosome] 14 because the chromosomal translocation t(11;14)(q13;q32) is found in some atypical CLL cases. If the break-apart probe for chromosome 14 is positive, a CCND1 translocation test can be performed. You could also look at a blot from the bone marrow for [CCND1] expression to rule out mantle cell lymphoma. Another aspect about the 11q deletion is that it associates with aggressive presentations, such as large bulky lymphadenopathy. Typically, this used to be associated with poor response rates for chemotherapy.
Whereas now, when you consider all the studies with Bruton tyrosine kinase [BTK] inhibitors, patients may have improved outcomes [progression-free survival (PFS)]. It’s unclear why this phenomenon [improved outcomes for patients with an 11q deletion treated with BTK inhibitors] is being observed, but [the 11q deletion] is still predictive of a high-risk feature at the time of diagnosis. For example, in this case, it is a possible explanation for the relatively rapid disease progression.

What are the guidelines for a patient like this?
The National Comprehensive Cancer Network [NCCN] guidelines for CLL without 17p deletion or mutations in TP53 include acalabrutinib [Calquence] [with or without] obinutuzumab [Gazyva], an anti-CD20 antibody, as a category 1 treatment regimen based on the ELEVATE CLL TN study [NCT02475681].3,4 Ibrutinib [Imbruvica] is another category 1 regimen for CLL based on the RESONATE-2 study [NCT01722487].5 It can be combined with obinutuzumab for frail patients [iLLUMINATE study (NCT02264574)6; category 2B], and rituximab [Rituxan] for young patients [ECOG-E1912 study (NCT02048813)7; category 2B]. The combination of venetoclax [Venclexta] and obinutuzumab is also a category 1 regimen. Fewer studies have been done in patients who are younger than 65 and without comorbidities. However, since these regimens have improved outcomes and decreased toxicity, they have become category 1 indications for these patients as well.
What is the evidence for the use of ibrutinib to treat patients with CLL?
The phase 3 RESONATE-2 study investigated the use of ibrutinib as a first-line therapy to treat CLL.8 The study enrolled patients with treatment-naive CLL/SLL [small lymphocytic lymphoma], patients with active disease who were 65 years or older, or had comorbidities. Patients with a 17p deletion were excluded. Patients were randomized 1:1 to receive ibrutinib [420 mg once daily] until progression or chlorambucil [Leukeran] for up to 12 cycles. It is important to note that updates for the 7-year follow-up for this study as well as for the ECOG-E1912 study [NCT02048813] will be published soon. The 5-year follow-up [for RESONATE-2] showed a 5-year PFS of 70%.
This is the furthest out that any patients treated with targeted agents in a frontline setting have been followed. Overall, this is an extraordinarily durable effect. Many of the progressions occurred in patients who stopped taking ibrutinib because of drug toxicity. True progressions on drug were relatively rare. There are patients who do progress over time, but for the most part these patients do extraordinarily well. It is remarkable and many older patients may not need additional agents if they stay on the drug. The downside is that it involves continual treatment and the cost, but so far, the data support long-term use.
This study is an example where patients with an 11q deletion seem to have a better PFS than those without this deletion.9 However, this is only a trend in the data. Since the study was not designed to have this as a primary or secondary end point, you can’t assess this trend statistically from these data. But meta and retrospective analyses have shown that this is a significant factor for people having good outcomes. So this is something I consider when deciding how to treat older patients who have bulky lymphadenopathies. On the other hand, the IGHV status, while important for assessing time to first treatment, does not seem to have prognostic significance when treating patients with BTK inhibitors. This is the case with this study where there was no difference in outcomes based on the IGHV mutational status, which is a paradigm shift away from chemotherapy, where there was a clear difference in PFS.
How do the BTK inhibitors compare with chemotherapy?
The ECOG 1912 was a phase 3 trial that compared ibrutinib plus rituximab to FCR [fludarabine (Fludara) plus cyclophosphamide (Cytoxan) plus rituximab].10 At the 48-month follow-up, the 3-year PFS rate was 89% for ibrutinib plus rituximab and 71% for FCR [HR, 0.39; 95% CI, 0.26-0.57]. OS at the 34-month follow-up was also modestly higher for the ibrutinib arm [98.8% vs 91.5%, respectively]. We will have to wait and see whether these values hold over time as patients continue to be salvaged with targeted agents as they relapse. A comparison of the effectiveness of the 2 therapy combinations based on IGHV mutational status showed that much of the benefit of ibrutinib was restricted to the unmutated group. There was no statistically significant difference between the 2-therapy combination for the IGHV-mutated group. These results support the argument that FCR can possibly be reserved for patients with IGHV mutations.
The ELEVATE TN study compared acalabrutinib with/ without obinutuzumab versus obinutuzumab plus chlorambucil in a similar population to the previous studies [treatment-naive, age ≥ 65 or < 65 years with coexisting conditions, cumulative illness rating scale [CIRS] score > 6 or creatinine clearance < 70 mL/min].7 This study did allow patients with a 17p deletion. Both acalabrutinib arms had better results than the chlorambucil arm. Interestingly, at the 28.3-month follow-up, there seemed to be a small benefit of adding obinutuzumab to acalabrutinib [HR, 0.10 (95% CI, 0.06-0.17) vs HR, 0.20 (95% CI, 0.13-0.30) for the monotherapy arm]. We’ll be able to see whether these results change at the next update. However, because this was not a primary or secondary end point for this study, we cannot say whether this difference is statistically significant. There was also no OS benefit between these arms.
The subgroup PFS analysis of the ELEVATE TN study also showed that there was a clear benefit in using acalabrutinib vs chlorambucil in low- and high-risk patients.11 This analysis shows that there seems to be an added benefit to the addition of obinutuzumab. This was especially true for patients with an 11q deletion and those with mutated IGHV, supporting the use of BTK inhibitors.
Ultimately, these high-risk features become irrelevant when using targeted therapies. The overall response rate [ORR] is a useful parameter to consider in CLL. However, the use of continuous response BTK inhibitors means that the response deepens over time. In the ELEVATE TN study, the ORR and the complete response [CR] rate were higher with the addition of obinutuzumab.12 It will be interesting to see at the update whether this changes and the acalabrutinib plus obinutuzumab group starts to look more like the monotherapy group, which plateaus at around 30% CR rate at 5 years. There was no difference between the 3 arms in terms of OS. I think the days of seeing OS benefits like with the RESONATE [NCT01578707] and RESONATE-2 studies, before venetoclax and other new agents and CAR [chimeric antigen receptor] T-cell therapy were approved, are probably over because people are going to be salvaged with the next lines of therapy and crossed over.
How does the modest benefit seen with obinutuzumab change what you do in your practice?
I think we saw a similar phenomenon in the comparison between bendamustine plus rituximab [BR] versus bendamustine plus obinutuzumab. That is, obinutuzumab showed an improved PFS for the treatment of follicular lymphoma but no OS benefit. However, we still use BR a lot despite these results. Obinutuzumab is associated with cytopenia, which complicates the regimen and raises the question of what the real benefit to the patients is. Again, it will be interesting to see the follow-up data and whether this can change practice at all if this continues to remain significant over time.
How do the BTK inhibitors compare in terms of safety?
In terms of safety, atrial fibrillation [A-fib] rates are lower with acalabrutinib than with ibrutinib. Any grade A-fib was at about 3% in the ELEVATE TN study, compared with 7% or 8% in the ibrutinib studies. There is also maybe a little less bleeding, hypertension, and other adverse events [AEs] reported in the ELEVATE TN study.13 So it is possible that acalabrutinib is less toxic, even when combined with obinutuzumab. There were reports of infusion reactions as well as tumor lysis syndrome, which is something to keep in mind. But for the most part, obinutuzumab does not add too much toxicity to the regimen and acalabrutinib seems to be very safe and effective, maybe more so than ibrutinib. The ELEVATE Relapse and Refractory study [NCT02477696] compared ibrutinib with acalabrutinib in previously treated patients with high-risk CLL.14
What is the evidence for the use of fixed-duration regimens of BTK inhibitors for CLL?
The CLL14 study [NCT02242942] was a phase 3, fixed-duration trial that compared venetoclax plus obinutuzumab versus chlorambucil plus obinutuzumab with 12 cycles total in both arms.15 This study had a similar patient population to the previous ones: older patients with a CIRS score greater than 6. The 4-year PFS rate was 74% for venetoclax plus obinutuzumab, which compares favorably with continual therapy with ibrutinib [70% at 5 years]. The minimal residual disease [MRD] rate was about 57% and MRD-negative patients did better and had more sustainable remission rates. There is a clear difference in the PFS rate based on the IGHV mutation status, where patients with IGHV unmutated progress sooner.
However, if the median PFS for patients with IGHV unmutated is not reached at year 4 or 5, it is unclear how we proceed in the clinic. Would these patients benefit from continued therapy, instead of a fixed-duration regimen? I am not sure. We do not have data to show that yet. We don’t have good retreatment data to see whether these patients can be salvaged with another line of therapy a few years later or with another round of venetoclax. So we may decide that patients with unmutated IGHV don’t get a fixed duration approach. That doesn’t mean that venetoclax isn’t useful for them, but that we don’t understand these data now.
What seems to be clear is that fixed duration regimens for high-risk patients [17p deletion, mutated TP53] don’t seem to be the best approach and these tumors deserve some continuation therapy. Again, that doesn’t mean that venetoclax is inappropriate for them. Venetoclax may get these patients to MRD negativity at high rates, which might be the best thing to do. You can eliminate high-risk clones and Richter’s transformations.
However, we know that the clonal growth of high-risk clones is higher than the non-high-risk clones. So even in an MRD-negative state, they grow back faster, suggesting that fixed-duration regimens are not ideal. Typically, I use BTK inhibitors for patients with 17p deletions in the frontline setting versus a fixed duration venetoclax-based approach.
Undetectable MRD in the peripheral blood was 76% and in the bone marrow it was 57% with values in the 10-6 ranges, indicating very deep remissions, which bodes well.15 FCR data have shown that remission depth corresponds to longer remissions regardless of therapy used. Therefore, increasing remission depth is going to be very important.
Overall, venetoclax plus obinutuzumab is a very safe combination. Cytopenia, neutropenia, and thrombocytopenia are common AEs and are mostly an effect of obinutuzumab because they tend to improve at the end of the study period when venetoclax is given as a monotherapy.
Fortunately, these cases of grade 3 and 4 neutropenia [53%] do not translate into febrile neutropenia [5%]. Just be aware of it and treat it aggressively.

Would you test for MRD?
The iwCLL guidelines recommend not to test outside of clinical trials. None of these clinical trials are based on MRD status. It’s prognostic only. I do test it just because… I can get the information and I can prognosticate for [patients]. If they’re MRD positive, you know not that it’s a standard thing to do, but sometimes I will continue data. It will deepen over time if patients are getting there, but again, it can confuse the matter and there is low-risk p13 depletion risk patients mutated who may have very low levels of MRD that you can stop them, and they probably are going to go without true progression for a long time. So, these things are not recommended in clinical practice. It looks like most people aren’t and until we have some adapted algorithm, we really can’t be introducing that.

Discuss your experience of managing A-Fib in patients treated with a BTK inhibitor.
Most groups consult with a cardiology team [and manage the patients together]. Some patients have been on BTK inhibitor therapy between 2 and 4 years and are in a deep remission and doing well. Therefore, the consensus is to continue treatment and manage the A-fib. In my practice, when a patient is on a BTK inhibitor and is newly diagnosed with rate-controlled A-fib, I keep them on the BTK inhibitor. If they start to have recurrent problems or hospitalizations, I try to switch the BTK inhibitor.
I give all patients a dose-hold for 1 or 2 weeks and many times the patients will go back to regular sinus rhythm because they are then on a β-blocker. Sometimes that prevents a recurrence. I haven’t had much luck with reducing the BTK inhibitor dose and preventing A-fib. Instead, I switch the class of agents because I think keeping patients on a full dose to improve the BTK occupancy is important.
Would you consider switching to zanubrutinib [Brukinsa]?
There is a head-to-head comparison between zanubrutinib and ibrutinib that showed a 5% vs 15% rate of A-fib, respectively. It does appear that the risk of A-fib may be lower with zanubrutinib. There are also phase 2 studies with acalabrutinib showing a 9.5% risk, 16% with ibrutinib.15 So I think [these new agents] probably do have lower rates of A-fib. I think zanubrutinib and acalabrutinib are relevant drugs. It would be good to see a head-to-head comparison between the 2 to really understand what the true difference is and whether there is a best drug in class.
How often are you seeing hypertension develop in your patients receiving ibrutinib?
I see a lot of it, and it is hard to convince the patients that it is a problem and to get them to see their primary care physicians to address it. I frequently start therapy to control the hypertension, sometimes 3 to 9 months after symptoms first appear. There’s no preferred agent. The pathophysiology behind this phenomenon is not well understood. I do think that later generation, better tolerated agents will have lower hypertension cases, based on phase 2 studies.
References:
1. Hallek M, Cheson BD, Catovsky D, et al. Guidelines for the diagnosis and treatment of chronic lymphocytic leukemia: a report from the International Workshop on Chronic Lymphocytic Leukemia updating the National Cancer Institute-Working Group 1996 guidelines [published correction appears in Blood. 2008;112(13):5259]. Blood. 2008;111(12):5446-5456. doi:10.1182/ blood-2007-06-09390
2. International CLL-IPI working group. An international prognostic index for patients with chronic lymphocytic leukaemia (CLL-IPI): a meta-analysis of individual patient data. Lancet Oncol. 2016;17(6):779-790. doi:10.1016/ S1470-2045(16)30029-8
3. NCCN. Clinical Practice Guidelines in Oncology. Chronic lymphocytic leukemia/ small lymphocytic lymphoma, version 4.2021. Accessed July 15, 2021. https:// www.nccn.org/professionals/physician_gls/pdf/cll.pdf
4. Sharman JP, Banerji V, Fogliatto LM, et al. ELEVATE TN: Phase 3 study of acalabrutinib combined with obinutuzumab (O) or alone vs O plus chlorambucil (Clb) in patients (Pts) with treatment-naive chronic lymphocytic leukemia (CLL). Blood. 2019;134(suppl 1):31. doi:10.1182/blood-2019-128404
5. Burger JA, Tedeschi A, Barr PM, et al; RESONATE-2 investigators. Ibrutinib as initial therapy for patients with chronic lymphocytic leukemia. N Engl J Med. 2015;373(25):2425-2437. doi:10.1056/NEJMoa1509388
6. Moreno C, Greil R, Demirkan F, et al. Ibrutinib plus obinutuzumab versus chlorambucil plus obinutuzumab in first-line treatment of chronic lymphocytic leukaemia (iLLUMINATE): a multicentre, randomised, open-label, phase 3 trial [published correction appears in Lancet Oncol. 2019;20(1):e10]. Lancet Oncol. 2019;20(1):43-56. doi:10.1016/S1470-2045(18)30788-5
7. Shanafelt TD, Wang XV, Kay NE, et al. Ibrutinib-rituximab or chemoimmunotherapy for chronic lymphocytic leukemia. N Engl J Med. 2019;381(5):432-443. doi:10.1056/NEJMoa1817073
8. Burger JA, Barr PM, Robak T, et al. Long-term efficacy and safety of first-line ibrutinib treatment for patients with CLL/SLL: 5 years of follow-up from the phase 3 RESONATE-2 study. Leukemia. 2020;34(3):787-798. doi:10.1038/ s41375-019-0602-x
9. Kipps TJ, Fraser G, Coutre SE, et al. Long-Term Studies Assessing Outcomes of Ibrutinib Therapy in Patients With Del(11q) Chronic Lymphocytic Leukemia. Clin Lymphoma Myeloma Leuk. 2019 Nov;19(11):715-722.e6. doi: 10.1016/j. clml.2019.07.004
10. Shanafelt TD, Wang V, Kay NE, et al. Ibrutinib and rituximab provides superior clinical outcome compared to FCR in younger patients with chronic lymphocytic leukemia (CLL): extended follow-up from the E1912 Trial. Blood. 2019;134(suppl 1):33. doi:10.1182/blood-2019-126824
11. Sharman JP, Egyed M, Jurczak W, et al. Acalabrutinib with or without obinutuzumab versus chlorambucil and obinutuzumab for treatment-naive chronic lymphocytic leukaemia (ELEVATE TN): a randomised, controlled, phase 3 trial. Lancet. 2020;395(10232):1278-1291. doi:10.1016/S0140-6736(20)30262-2
12. Byrd JC, Woyach JA, Furman RR, et al. Acalabrutinib in treatment-naive chronic lymphocytic leukemia. Blood. 2021;137(24):3327-3338. doi:10.1182/ blood.2020009617
13. Byrd JC, Hillmen P, Ghia P, et al. First results of a head-to-head trial of acalabrutinib versus ibrutinib in previously treated chronic lymphocytic leukemia. J Clin Oncol. 2021;39(suppl 15):7500. doi:10.1200/JCO.2021.39.15_suppl.7500
14. Fischer K, Al-Sawaf O, Bahlo J, et al. Venetoclax and obinutuzumab in patients with CLL and coexisting conditions. N Engl J Med. 2019;380(23):2225‐2236. doi:10.1056/NEJMoa1815281
15. Al-Sawaf O, Zhang C, Tandon M, et al; CLL14 Study Investigators. Fixed-duration venetoclax-obinutuzumab for previously untreated patients with chronic lymphocytic leukemia: follow-up of efficacy and safety results from the multicenter, open-label, randomized phase III CLL14 trial. J Clin Oncol. 2020;38(suppl 15):8027. doi:10.1200/JCO.2020.38.15_suppl.8027

Leslie Reviews Relevant Data for Treatment of Relapsed/Refractory CLL
May 8th 2024During a Case-Based Roundtable® event, Lori A. Leslie, MD, discussed Bruton tyrosine kinase inhibition options for a patient with relapsed/refractory chronic lymphocytic leukemia in the first article of a 2-part series.
Read More
Overcoming Barriers in Cancer Clinical Trials: A Path Forward for Better Patient Care
April 29th 2024Clinical trials play a pivotal role in developing effective therapies, yet their integration is challenged by issues such as insufficient reimbursement structures, misaligned incentives, physician burnout, and a complex regulatory environment.
Read More