April Roundtable Roundup: B-cell Lymphoma
In separate, live virtual events, Kami J. Maddocks, MD, and Sairah Ahmed, MD, discussed the use of loncastuximab tesirine for a patient with later-line diffuse large B-cell lymphoma.
CASE SUMMARY
- A 73-year-old woman presented with fever, headaches, and a 7-lb unintentional weight loss .
- Family history: married with 2 grown children who live in other states; primary caretaker for her mother who has advanced dementia; no family history of cancer
- Medical history: hyperlipidemia well controlled with simvastatin
- Physical exam: palpable bilateral cervical lymphadenopathy
- Laboratory results: lactate dehydrogenase, 265 U/L (upper limit, 280 U/L); hemoglobin, 10.8 g/dL; bilirubin, 1.3 mg/dL (upper limit, 1.2 mg/dL); creatinine, 1.7 mg/dL (upper limit, 1.2 mg/dL); all others within normal limit
- Hepatitis B, C, and HIV negative
- Lymph node biopsy; immunohistochemistry panel: CD10+, CD20+, which confirmed diffuse large B-cell lymphoma (DLBCL)
- Fluorescence in situ hybridization: negative for rearrangements of BCL6, BCL2, and C-MYC
- Whole body PET/CT scan showed diffuse adenopathy, largest node 3.9 cm
- MRI of the brain showed no evidence of lesions
- ECOG performance status: 1
- Stage III disease; International Prognostic Index score 2; low-intermediate risk
- The patient received 6 cycles of R-CHOP (rituximab [Rituxan], cyclophosphamide, doxorubicin hydrochloride, and vincristine sulfate [Oncovin], and prednisone), which she tolerated well.
- She had a complete response (CR) at the end of treatment, but 14 months later she presented with diffuse lymphadenopathy, confirmed by PET/CT scan .
- Biopsy showed relapse of DLBCL
- The patient received 6 cycles of polatuzumab vedotin (Polivy), bendamustine, and rituximab (Pola-BR).
- She achieved response, then progressed 8 month s later.
- The patient is not able to travel because she is the primary caretaker for her mother, who has dementia.
- Current treatment: loncastuximab tesirine-lpyl (Zynlonta)
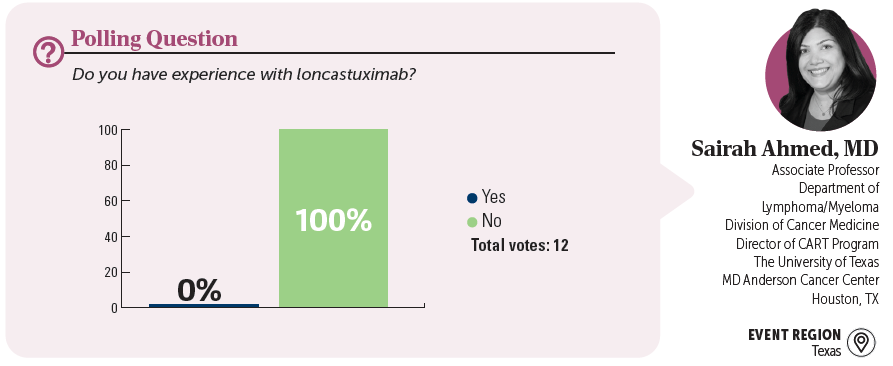
EVENT REGION Kentucky, Michigan, Illinois, Indiana, Ohio, and Wisconsin
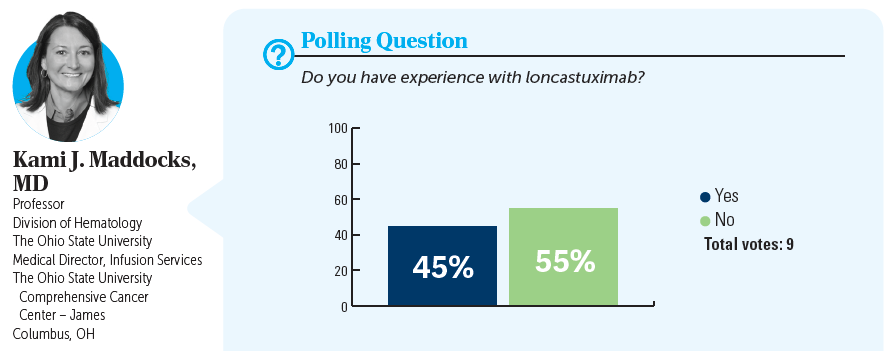
KAMI J. MADDOCKS, MD: Do you agree with this treatment choice? Who has heard of or used loncastuximab?
MOHAMAD K. KHASAWNEH, MD: This is a scenario that I would seriously consider loncastuximab for because the patient seems to know that treatment is palliative at this time. She’s not looking into any intensive treatment with the hope of cure. She has a difficult social situation of taking care of her mom. I have used single-agent loncastuximab [before], and it’s definitely a great option at this time because of its mechanism of action. It does have its own adverse event [AE] profile, like fluid overload, fatigue, and some myelosuppression, [although] not as high as we see with chemotherapy or tafasitamab-cxix [Monjuvi] and lenalidomide [Revlimid].
NEERAJ MAHAJAN, MD: I have used loncastuximab and it turned out to be the most practical and logistically feasible single agent, given every 3 weeks, 30 to 45 minutes infusion, with no premedications, so you don’t have to combine it with BR and you don’t have to get [patients] approved for lenalidomide. AEs are there, but compared with other options, I think they are still manageable. At least for a lot of these patients who have these issues with travel and comorbidities, that is a reasonable choice; at least in the community setting and from the scheduling and doing the infusions for those patients, it is the most convenient of [the treatment options].
MARK H. KNAPP, MD: I’ve also used it. It seems very convenient for the patient every 3 weeks—a short infusion. The patient I had tolerated it, is on it currently, and tolerated it much better than their second-line therapy.

Overcoming Barriers in Cancer Clinical Trials: A Path Forward for Better Patient Care
April 29th 2024Clinical trials play a pivotal role in developing effective therapies, yet their integration is challenged by issues such as insufficient reimbursement structures, misaligned incentives, physician burnout, and a complex regulatory environment.
Read More
Gholam Analyzes Treatment Outcomes for Advanced HCC in Child-Pugh B Population
April 28th 2024During a live Community Case Forum event in partnership with the Tennessee Oncology Practice Society, Pierre Gholam, MD, examined the current state of treatment for patients with hepatocellular carcinoma, looking in particular at what data is available for those with Child-Pugh B and C status who have poorer outcomes and have limited data from prospective clinical trials.
Read More