Kaklamani Discusses Approach to CDK4/6 Inhibitor and Later Therapy for ER+ Breast Cancer
During a Targeted Oncology™ Case-Based Roundtable™ event, Virginia Kaklamani, MD, discussed with participants the case of a patient with estrogen receptor–positive breast cancer who showed signed of progression 20 months after treatment with letrozole plus palbociclib.
Virginia Kaklamani, MD (MODERATOR)
Professor of Medicine
Division of Hematology/Oncology
UT Health San Antonio
San Antonio, TX

EVENT REGION Arkansas, Missouri, and Texas
PARTICIPANT LIST Belal Firwana, MD | Yifan Tu, MD | Steve Rosenfield, MD | Nishant Poddar,MD | Eric Schaefer, MD | Raymond Lobins, DO | Marcela Mazo Canola, MD | Christiane Zoghbi, MD | Somasekhar Bandi, MD
CASE SUMMARY
A 56-year-old postmenopausal woman presented with a palpable right breast mass with no clinically abnormal axillary lymph nodes. A core biopsy led to a diagnosis of grade 2, estrogen receptor–positive (ER+)/progesterone receptor–positive (PR+) invasive ductal carcinoma (IDC) with a HER2 immunohistochemistry (IHC) score of 0 and Ki-67 score of 33%. Lumpectomy and sentinel lymph node (SNL) biopsy results showed a tumor measuring 1.8 cm, grade 2 IDC, and 2 SNLs negative for malignant cells. Her 21-gene recurrence score was 27.
The patient received docetaxel (Taxotere) and cyclophosphamide (Cytoxan) for 4 cycles followed by radiation therapy, and she completed 5 years of adjuvant aromatase inhibitor (AI) therapy.
Three years later, she reported right-sided abdominal pain and mild nausea. A CT scan of the chest, abdomen, and pelvis showed 3 suspicious liver lesions (right lobe; largest measuring 2 cm). Liver biopsy results showed ER+/PR+ IDC with a HER2 IHC score of 0. Comprehensive molecular testing results from tissue biopsy showed no actionable alterations. AI therapy with letrozole (Femara) plus palbociclib (Ibrance) was initiated.
The therapy was well tolerated by the patient, with grade 2 neutropenia that did not require dose modification. Twenty months later, follow-up imaging showed enlargement of liver nodules and 2 new lung nodules (largest measuring 0.9 cm). Her ECOG performance status is 0. Blood-based circulating tumor DNA analysis showed an ESR1 mutation.
DISCUSSION QUESTIONS
- Which CDK4/6 inhibitor are you using most often as first-line therapy?
- Do the data from the MAINTAIN (NCT02632045) and/or PACE (NCT03147287) studies influence your practice in any way?
FIRWANA: I tend to use abemaciclib [Verzenio], because [although] it has more diarrhea than the others, overall, it has less neutropenia.1 And I feel overall, it’s kind of easy to follow. It’s every day without on/off [dosing]. I haven’t changed my practice since the introduction of ribociclib [Kisqali], but I would probably use it more.
KAKLAMANI: Is that because of the overall survival [OS] data [from MONALEESA-2 (NCT01958021)]?2
FIRWANA: Correct.
TU: I use ribociclib.
KAKLAMANI: Were you always using ribociclib, or did you switch based on the OS data?
TU: I always used ribociclib.
ROSENFIELD: Palbociclib was the first to market and so the argument for palbociclib [despite] not having OS data is that their trials had more heavily treated patients in later lines of therapy. Do you find that true? Or do you just feel that the other 2 are superior to palbociclib because palbociclib doesn’t have the OS advantage?
KAKLAMANI: It’s hard to do cross-trial comparisons; we’re always taught not to.
Second, for all of these trials the primary end point was progression-free survival [PFS]; it wasn’t OS. I feel it’s a little unfair…that we’re judging a drug on OS, based on the fact that [the PALOMA-2 (NCT01740427)] trial did have more patients with high-volume disease, and also the OS was a secondary end point. What swayed me to start using ribociclib more was the fact that it wasn’t just the metastatic trials; it was also [negative results from] the PALLAS adjuvant trial [NCT02513394]. You [may] make the argument that they included lower-risk patients and there’s a high discontinuation rate from the palbociclib, and so maybe that’s why we didn’t have significant results.3
But when you look at all of the evidence that we have, we have ribociclib, which has OS data in the first line and the subsequent lines. We have abemaciclib, which has survival data in the subsequent lines, and [has] survival data in the first line [which showed over 13 months improvement in median OS but no statistically significant improvement].4
We have adjuvant trials with both ribociclib and abemaciclib that have an improvement in [invasive disease-free survival].5,6 So that’s why I have switched. But I think it’s absolutely reasonable to give palbociclib. The patients for whom I have already started palbociclib, I have not switched any of them to ribociclib. As far as toxicity, it seems to be the easiest one.
PODDAR: Does tumor burden, particularly location of tumor burden, like liver or bone metastasis, or visceral metastasis apart from the liver, determine which out of the 3 CDK4/6 inhibitors you use?
KAKLAMANI: It doesn’t matter. I will use a [particular] CDK4/6 inhibitor regardless. I’m not going to think one is better than the other [based on location alone]. What I would potentially do is if I have somebody who is a little older, I would be a little concerned about them. I may not use abemaciclib if I’m afraid of diarrhea. I might use palbociclib because in my [experience] it has been a little bit easier to give them that than ribociclib. But that’s been the exception, not the rule. I feel that all 3 are pretty easy to give in general. With abemaciclib now given enough in the adjuvant setting, we’ve figured out how to give it without any issues.
One of the things to keep in mind is whether every single patient is eligible for a CDK4/6 inhibitor in the first-line setting. Are there some who we might be able to spare from giving a CDK4/6 inhibitor? There were some data presented at the 2023 American Society of Clinical Oncology Annual Meeting that were interesting, suggesting that we may be able to spare some patients and give them a CDK4/6 inhibitor in the second line.7 We just have to make sure that those patients have low enough burden of disease that we will get to that second line. We all know that some patients, they only get 1 line of therapy and then after that they progress rapidly. Something happens, their performance status declines, and they end up going to hospice. Because of the great data in both PFS and OS, we don’t want somebody to not be exposed to a CDK4/6 inhibitor.
SCHAEFER: With ribociclib’s adjuvant dose being 400 mg, are you starting your patients with metastatic disease at 400 mg or are you still doing 600 mg and dose titrating down if necessary?
KAKLAMANI: I’m still starting with 600 mg and dose titrating. There were nice data presented for all of the CDK4/6 inhibitors showing that dose de-escalation did not impact efficacy.8,9 I’m very comfortable dose reducing these patients in the adjuvant setting as well because I think, like with most of our drugs, we’re overdosing.
LOBINS: When I used to see these patients and still to this day, if they have a lot of visceral disease, I would be thinking sometimes they need chemotherapy instead of hormonal therapy. There was a recent trial that looked at hormonal therapy and CDK4/6 inhibitors even in patients with visceral disease and they did just as good as the chemotherapy. Has that changed your practice? What do you do now with patients with extensive disease?
KAKLAMANI: That was the Taiwanese study; they were very brave and they did this trial that we could not do in the United States, and kudos to them. They showed exactly that patients receiving the CDK4/6 inhibitors may have even had better outcomes compared with chemotherapy.10 It has made me feel more strongly about giving endocrine therapy in the first line. If I see somebody whose tumor has ER expression of 20%, and PR expression is 5%, that’s a different story. But for the patients who have relatively strong ER/PR status, and I feel that their tumor is endocrine sensitive, it will be extremely unlikely for me to give them chemotherapy in the first line.
MAZO CANOLA: What are your thoughts on those patients who progressed on adjuvant abemaciclib?
KAKLAMANI: That’s another great point. This is a data-free zone. I haven’t had a patient like this yet. I think we’re going to start seeing less metastatic disease because of these [adjuvant] data, and that’s great. I would switch them to a different CDK4/6 inhibitor, though. I would probably give them ribociclib, and the data from MAINTAIN help me do that. If a patient were on a year of abemaciclib and then developed metastatic disease, I’d think about it twice. But if they’ve been on a couple of years, and then a year or 2 later develop metastatic disease, I’d be more than happy to rechallenge them with the CDK4/6 inhibitor.
DISCUSSION QUESTIONS
- Have you used the oral selective estrogen receptor degrader (SERD) elacestrant (Orserdu) for your patients with breast cancer?
- What efficacy and/or safety data most impact your decision to prescribe oral SERDs—approved or investigational?
ROSENFIELD: I have 2 patients on [elacestrant], and it’s too early to tell for efficacy, but they’re tolerating extremely well.
KAKLAMANI: Perfect. Do they have any gastrointestinal [GI] adverse events [AEs] at all?
ROSENFIELD: No. I’m waiting and I’m asking [the patient], but so far, so good.
ZOGHBI: I also used it but didn’t have very good response; the patient progressed after 3 months. The patient didn’t develop toxicity but she did complain that the [345-mg tablet] was too big. She would not swallow it or take it so finally we were able to find another dose of 84-mg tablets. She was willing to take that. I thought that’s interesting for an oral medicine.
KAKLAMANI: Yes, that is interesting. Initially, the formulation was a capsule and it caused a lot of GI toxicity, [in particular] caused a lot of GERD [gastroesophageal reflux disease]. The company switched the formulation from the capsule to the tablet, and the GERD pretty much went away. But that’s interesting about the size.
BANDI: I’ve used it with 1 patient but she progressed. She tolerated it OK, but it was sad that she could not get any response. I had to change treatment.
DISCUSSION QUESTIONS
- Do the EMERALD trial (NCT03778931) data on duration of CDK4/6 inhibitor treatment influence your practice for the ESR1-mutated population or the dual mutation ESR1/PI3K subgroups?
- How do the safety data from EMERALD compare with standard therapies used in the second-line and third-line settings (eg, everolimus [Afinitor] plus exemestane [Aromasin] or alpelisib [Piqray] plus fulvestrant [Faslodex])?
ROSENFIELD: From an AE standpoint, I think the elacestrant is better tolerated than alpelisib or everolimus, so all things equal, I would tend to go with elacestrant first.
KAKLAMANI: Yes, that makes sense. It does have a better AE profile.11
LOBINS: I would want to [identify] the two-thirds of patients who are not going to respond to endocrine therapy. Because when you look at second-line treatment with a CDK4/6 inhibitor, the PFS was about a year. And in this trial, the 6-month PFS rate was 34% and 12-month PFS rate was 22%, which is great [From the Data11], but weeding out the rest of those would help your patients a lot in knowing which direction to go.
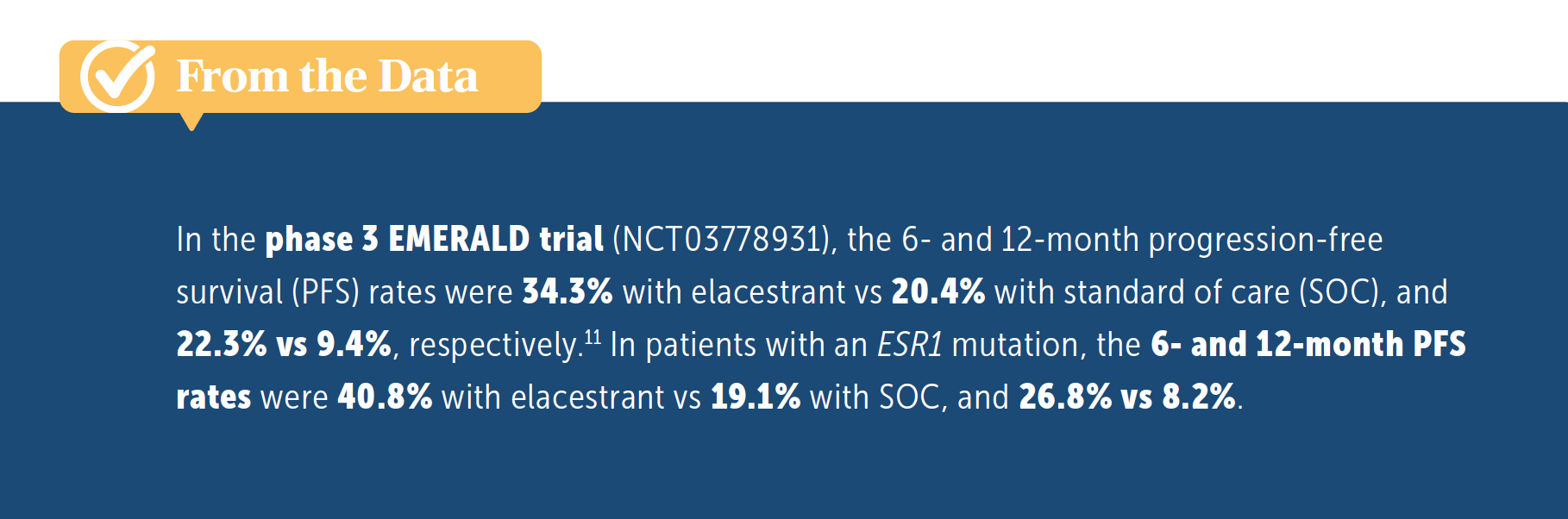
KAKLAMANI: Yes, I think that’s a million-dollar question right now. When we looked at EMERALD, we looked at every dataset to try to see if there’s some clinical characteristic that told us who the patients were that would have rapid progression of disease and who weren’t. If you look at the post CDK4/6 inhibitor data with everolimus or alpelisib, all of these drugs give you a median PFS of around 5 months.12,13 Single-agent endocrine therapy gives you a median PFS of 2 to 3 months.14 So when we’re talking about how successful we are in treating ER-positive disease, especially in that first-line setting with CDK4/6 inhibitors, we’re pretty unsuccessful in that second-line setting. This is something that we need to be working on: identifying patients who still have endocrine-sensitive disease, or identifying drugs that will reverse that endocrine insensitivity, so that we can continue treating them with endocrine therapy. I think your comment is extremely important. Clinically, the only thing we have right now is that CDK4/6 inhibitor duration, which is by no means perfect.
REFERENCES
1. Goetz MP, Toi M, Campone M, et al. MONARCH 3: abemaciclib as initial therapy for advanced breast cancer. J Clin Oncol. 2017;35(32):3638-3646. doi:10.1200/JCO.2017.75.6155
2. Hortobagyi GN, Stemmer SM, Burris HA, et al. Overall survival with ribociclib plus letrozole in advanced breast cancer. N Engl J Med. 2022;386(10):942-950. doi:10.1056/NEJMoa2114663
3. Gnant M, Dueck AC, Frantal S, et al. Adjuvant palbociclib for early breast cancer: the PALLAS trial results (ABCSG-42/AFT-05/BIG-14-03). J Clin Oncol. 2022;40(3):282-293. doi:10.1200/JCO.21.02554
4. Toi M, Huober J, Sohn JH, et al. MONARCH 3: Final overall survival results of abemaciclib plus a nonsteroidal aromatase inhibitor as first-line therapy for HR+, HER2- advanced breast cancer. Presented at: San Antonio Breast Cancer Symposium; December 5-9, 2023; San Antonio, TX. Abstract GS01-12.
5. Johnston SRD, Harbeck N, Hegg R, et al. Abemaciclib combined with endocrine therapy for the adjuvant treatment of HR+, HER2-, node-positive, high-risk, early breast cancer (monarchE). J Clin Oncol. 2020;38(34):3987-3998. doi:10.1200/JCO.20.02514
6. Slamon DJ, Stroyakovskiy D, Yardley DA, et al. Ribociclib and endocrine therapy as adjuvant treatment in patients with HR+/HER2- early breast cancer: Primary results from the phase III NATALEE trial. J Clin Oncol. 2023;41(suppl 17):LBA500. doi:10.1200/JCO.2023.41.17_suppl.LBA500
7. Sonke GS, Van Ommen-Nijhof A, Wortelboer Noor, et al. Primary outcome analysis of the phase 3 SONIA trial (BOOG 2017-03) on selecting the optimal position of cyclin-dependent kinases 4 and 6 (CDK4/6) inhibitors for patients with hormone receptor-positive (HR+), HER2-negative (HER2-) advanced breast cancer (ABC). J Clin Oncol. 2023;41(suppl 17):LBA100. doi:10.1200/JCO.2023.41.17_suppl.LBA1000
8. Hart LL, Bardia A, Beck JT, et al. Impact of ribociclib (RIB) dose modifications (mod) on overall survival (OS) in patients (pts) with HR+/HER2- advanced breast cancer (ABC) in MONALEESA(ML)-2. J Clin Oncol. 2022;40(suppl 16):1017. doi:10.1200/JCO.2022.40.16_suppl.1017
9. Burris HA, Chan A, Bardia A, et al. Safety and impact of dose reductions on efficacy in the randomised MONALEESA-2, -3 and -7 trials in hormone receptor-positive, HER2-negative advanced breast cancer. Br J Cancer. 2021;125(5):679-686. doi:10.1038/s41416-021-01415-9
10. El Saghir NS, Yap YS, Eralp Y, et al. Outcomes with first-line (1L) ribociclib (RIB) + endocrine therapy (ET) vs physician’s choice combination chemotherapy (combo CT) by age in pre/perimenopausal patients (pts) with aggressive HR+/HER2− advanced breast cancer (ABC): a subgroup analysis of the RIGHT Choice trial. J Clin Oncol. 2023;41(suppl 16):1063. doi:10.1200/JCO.2023.41.16_suppl.1063
11. Bidard FC, Kaklamani VG, Neven P, et al. Elacestrant (oral selective estrogen receptor degrader) versus standard endocrine therapy for estrogen receptor-positive, human epidermal growth factor receptor 2-negative advanced breast cancer: results from the randomized phase III EMERALD trial. J Clin Oncol. 2022;40(28):3246-3256. doi:10.1200/JCO.22.00338
12. Jacobson A. Alpelisib plus fulvestrant or letrozole demonstrates sustained benefits across subgroups of patients with PIK3CA-Mutated HR+/HER2- advanced breast cancer. Oncologist. 2022;27(suppl 1):S13-S14. doi:10.1093/oncolo/oyac011
13. Mo H, Renna CE, Moore HCF, et al. Real-world outcomes of everolimus and exemestane for the treatment of metastatic hormone receptor-positive breast cancer in patients previously treated with CDK4/6 inhibitors. Clin Breast Cancer. 2022;22(2):143-148. doi:10.1016/j.clbc.2021.10.002
14. Ashai N, Swain SM. Post-CDK 4/6 inhibitor therapy: current agents and novel targets. Cancers (Basel). 2023;15(6):1855. doi:10.3390/cancers15061855
Breast Cancer Leans into the Decade of Antibody-Drug Conjugates, Experts Discuss
September 25th 2020In season 1, episode 3 of Targeted Talks, the importance of precision medicine in breast cancer, and how that vitally differs in community oncology compared with academic settings, is the topic of discussion.
Listen
Batalini Explores Role of UGT1A1 in Patients Treated With Sacituzumab Govitecan for HR+ MBC
April 22nd 2024During a Community Case Forum live event in partnership with The Arizona Clinical Oncology Society, Felipe Batalini, MD, discussed the TROPiCS-02 trial of sacituzumab govitecan and the impact of the UGT1A1 status on adverse event frequency.
Read More
Novel Approaches Focus on Limiting Toxicity in Older Patients With ALL
April 22nd 2024The major challenges for clinicians treating older patients with acute lymphoblastic leukemia surround the emergence of resistance to existing therapies and the toxicities associated with current chemotherapies.
Read More
