Real-World Analysis of THP in mBC Shows Similar Survival, Safety Outcomes to CLEOPATRA Trial
Pertuzumab combined with trastuzumab, and docetaxel revealed comparable survival outcomes with previous studies of patients with metastatic breast cancer, including the CLEOPATRA trial.
A real-world analysis evaluating the triplet regimen of pertuzumab (Perjeta), trastuzumab (Herceptin), and docetaxel—THP—in Korean patients with metastatic breast cancer (mBC) revealed comparable survival outcomes with previous studies, including the CLEOPATRA trial (NCT00567190).1,2
THP has become the first-line standard of care for treating patients with HER2-positive mBC as a result of findings from CLEOPATRA.2 In the current study, after a median follow-up of 28.7 months, median overall survival (OS) and progression-free survival (PFS) were 58.3 months (95% CI, 36.6-80.0) and 19.1 months (95% CI, 16.2-21.9), respectively.
After an initial loading dose of 8 mg/kg of trastuzumab, patients received 6 mg/kg of trastuzumab, 420 mg of pertuzumab after an initial dose of 840 mg, and 75 mg/m2 of docetaxel every 3 weeks. The primary objective was to evaluate survival outcomes, including median OS and PFS. Secondary objectives were to assess treatment efficacy by objective response rate (ORR) and clinical outcomes of subsequent treatment after progression.
Investigators evaluated 228 patients with mBC who received THP. In 220 patients with measurable lesions, the ORR was 86.8% with a 17.7% complete response (CR) rate (n = 39/220; FIGURE1).
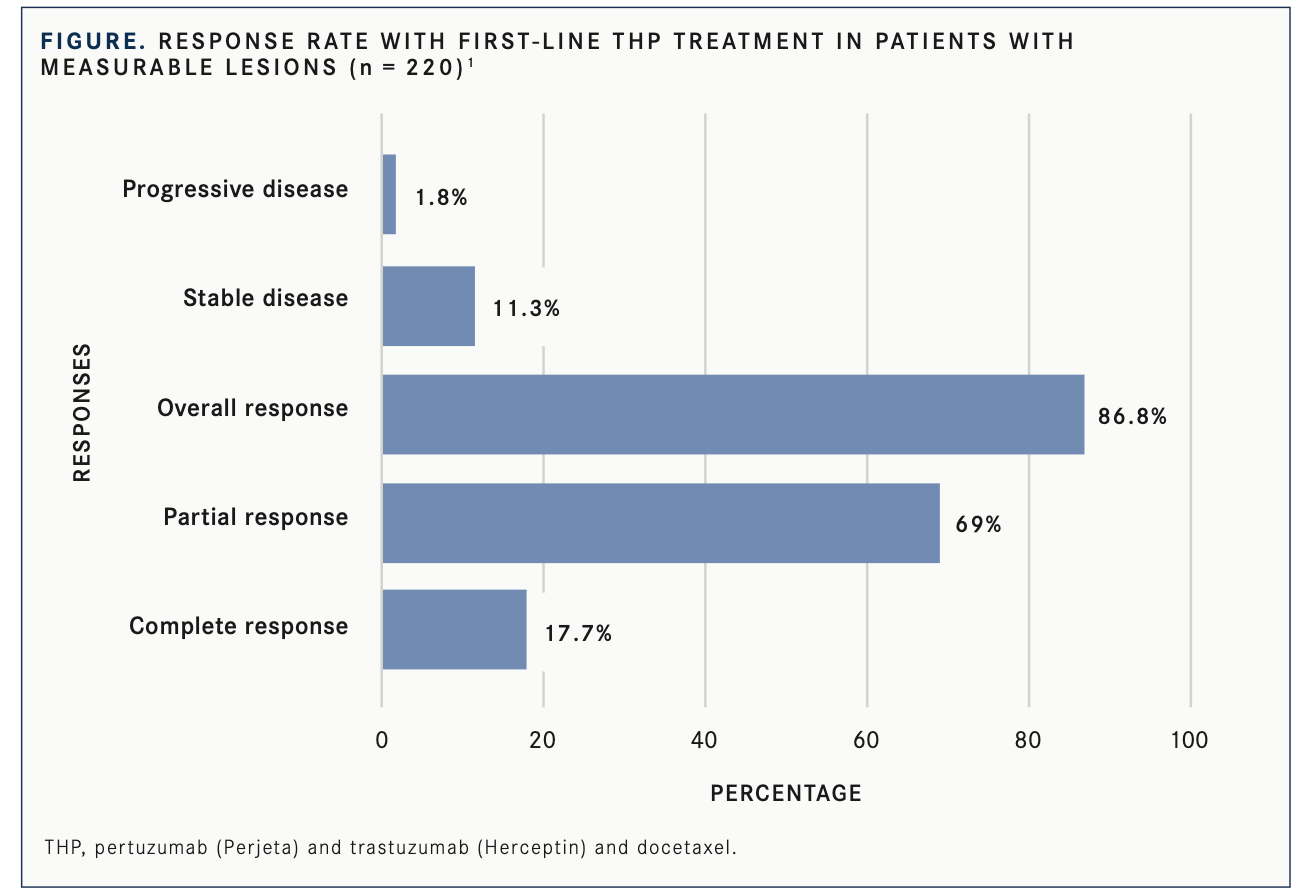
The median duration of response among patients who responded was 21.3 months (95% CI, 15.1-27.5); 93.1% of responders (n = 178/191) received docetaxel for more than 6 cycles. Among responders, 32 (16.7%) were long-term responders, defined as patients who sustained the THP regimen over 35 months.
Investigators reported that long-term responders had better survival outcomes and higher CR rates than non–long-term responders. Further, they observed more long-term responders among trastuzumab-naïve patients than in other patient groups.
Regarding toxicities, 118 patients (51.7%) experienced neutropenia of any grade and specifically, 27.6% of patients experienced grade 3 or 4 neutropenia. Investigators concluded that mBC is an effective first-line treatment with a good safety profile in a real-world context, consistent with findings from the CLEOPATRA trial.
References
1. Lee YP, Lee MS, Kim H, et al. Real-world evidence of trastuzumab, pertuzumab, and docetaxel combination as a first-line treatment for Korean patients with HER2-positive metastatic breast cancer. Cancer Res Treat. Published online January 17, 2022.doi:10.4143/crt.2021.1103
2. Swain SM, Baselga J, Kim SB, et al; CLEOPATRA Study Group.Pertuzumab, trastuzumab, and docetaxel in HER2-positive metastatic breast cancer. N Engl J Med. 2015;372(8):724-734. doi:10.1056/NEJMoa1413513
Breast Cancer Leans into the Decade of Antibody-Drug Conjugates, Experts Discuss
September 25th 2020In season 1, episode 3 of Targeted Talks, the importance of precision medicine in breast cancer, and how that vitally differs in community oncology compared with academic settings, is the topic of discussion.
Listen
Overcoming Barriers in Cancer Clinical Trials: A Path Forward for Better Patient Care
April 29th 2024Clinical trials play a pivotal role in developing effective therapies, yet their integration is challenged by issues such as insufficient reimbursement structures, misaligned incentives, physician burnout, and a complex regulatory environment.
Read More