Early-Phase Trial Data of EGFR Inhibitors Lead to Successful Use of Osimertinib in Resected EGFR+ NSCLC
Osimertinib has been a successful therapy for the treatment of EGFR-positive non–small cell lung cancer, surpassing the effects of chemotherapy. In he adjuvant setting, physicians questions when and how to use osimertinib.
Nathan A. Pennell, MD, PhD

Osimertinib (Tagrisso) has been a successful therapy for the treatment of EGFR-positive non–small cell lung cancer (NSCLC), surpassing the effects of chemotherapy. In he adjuvant setting, physicians questions when and how to use osimertinib.
During a Targeted Oncology Case-Based Peer Perspectives event, Nathan A. Pennell, MD, PhD, professor, Department of Medicine Member, Developmental Therapeutics Program, at Case Comprehensive Cancer Center and a medical oncologist, Department of Hematology and Oncology, Taussig Cancer Center at Cleveland Clinic Cancer Center, discussed osimertinib's success in this patient population.
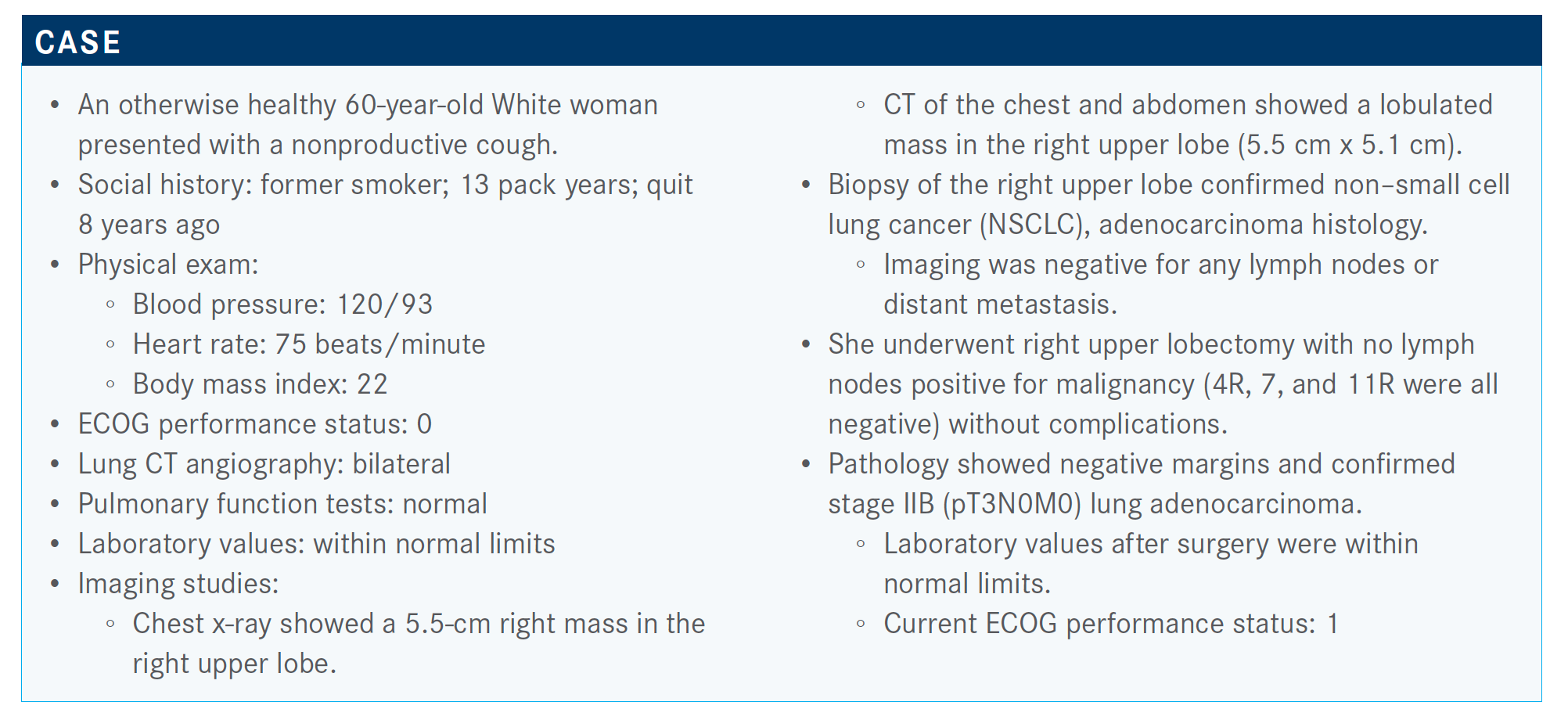
Targeted OncologyTM: Is there evidence for performing molecular testing in patients with early-stage NSCLC?
PENNELL: The College of American Pathologists andInternational Association for the Study of Lung Cancer guidelines recommend testing even earlier-stage patients, and that was before...evidence supporting adjuvant treatment, just because [there is] such a high rate of recurrence for higher-stage patients.. It’s useful to have that information and good to know.
How do you discuss adjuvant chemotherapy with a patient?
The consensus guidelines from the American Society of Clinical Oncology, National Comprehensive Cancer Network, and European Society for Medical Oncology state that there is evidence that adjuvant chemotherapy is not helpful in stage IA disease and may potentially be harmful. Stage IIA, IIB, and stage IIIA show a survival benefit to cisplatin-based chemotherapy. In the current staging system, where 4 cm or greater is now stage IIA disease, they still recommend considering stage IB for adjuvant chemotherapy if [a patient] has certain high-risk features.2
Which parameters are important when deciding whether to add adjuvant radiation therapy?
Up to this point, we have not had a good prospective randomized study looking at postoperative radiation specifically for N2-positive disease. Now we finally have one. There were retrospective data looking at patients in the ANITA 01 adjuvant trial [ISRCTN95053737]. Many of them received radiation, and investigators compared those who did and didn’t get radiation in the stage III patients.3 [Resources] like Cancer Immunology Research and National Cancer Database suggest that there may [be] a survival benefit to postoperative radiation.
The LUNG ART trial [NCT00410683], which was presented at the European Society for Medical Oncology [Virtual] Congress 2020, included patients who had fully resected NSCLC and were found to be N2-positive post operatively. After appropriate adjuvant chemotherapy, they were randomized to either the control, which was no radiation, or postoperative radiotherapy at 54 Gy. The primary end point was disease-free survival [DFS]. Results showed a numeric improvement in DFS, but this was not statistically significant. By 3 years, there was only about a 3% difference in DFS.4 Also, there was a preliminary overall survival curve, and those curves were overlapping. Although we have to wait for the final publication, this feels potentially practice changing for people who are routinely giving postoperative radiation.
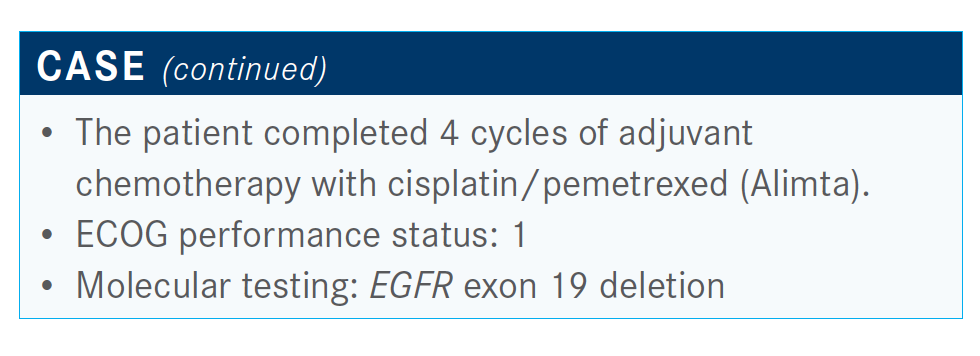
What is known about adjuvant EGFR-targeted therapy?
The SELECT trial, which was a single-arm study, showed a fairly high 2-year DFS [rate] with 2 years of adjuvant erlotinib [Tarceva].5 However, it was not randomized, so not too many conclusions could be drawn from it. A subgroup of patients with EGFR mutation–positive tumors from the RADIANT study [NCT00373425], which was in all-comer [NSCLC population], showed no benefit to adjuvant erlotinib. But in the EGFR-mutant subgroup, there was a significant difference in DFS for the 2 years of adjuvant erlotinib,6 although this was not found to be significant because it wasn’t one of the primary end points of the study and did not translate into an improvement in overall survival in this small subgroup.
Finally, there was a much better designed phase 3 study in [patients with EGFR mutations], the ADJUVANT study [NCT01405079], where patients were randomized to adjuvant vinorelbine plus cisplatin versus gefitinib [Iressa], the firstgeneration EGFR tyrosine kinase inhibitor [TKI] in EGFR-mutant lung cancer. The majority of patients on this trial had stage IIIA disease, so it was a fairly high-risk group. DFS was improved with 2 years of adjuvant gefitinib [28.7 months versus 18.0 months]. However, by 3 years there was no difference in DFS. Almost everyone—about 80% of the people—recurred by 3 years.7 There seems to be, at least in the short term, a delay or a reduction in early recurrences with adjuvant TKIs. But so far there hasn’t been a clear long-term benefit in terms of recurrences or survival.
What do data from the phase 3 ADAURA trial (NCT02511106) show?
ADAURA was presented at the 2020 American Society of Clinical Oncology Virtual Scientific Program. These were patients with stage IB, II, or IIIA NSCLC, all with common EGFR mutations. Patients could receive adjuvant chemotherapy as appropriate for their stage. They were then randomized to up to 3 years adjuvant osimertinib [Tagrisso], the third-generation EGFR-specific inhibitor, versus a placebo, with a primary end point of DFS in stage II and stage IIIA disease. Most of the patients were women, and two-thirds of them were nonsmokers. About two-thirds were Asian. There was an even split between IB, II, and IIIA disease.8,9
At 2 years, DFS was 90% versus 44%, [respectively], with a hazard ratio of 0.17 [95% CI, 0.12-0.23].9 This is as dramatic a difference in DFS as you could expect to see. Ninety percent of patients with DFS at 2 years is similar to what was seen in the previous EGFR-mutant lung cancer trials. The difference in this trial is that it’s osimertinib, which we consider a more potent and more tolerable central nervous system [CNS]–penetrant drug. Also, this was 3 years of treatment, whereas previous trials were only 2 years. The other thing to keep in mind is that there’s only about 22 months of follow-up. Almost everyone on this trial is still receiving adjuvant treatment, so we don’t know what’s going to happen when they stop.
The other message from this is that the recurrence rate is extremely high in the placebo group. You can see more than 50% of people have already recurred in the first 2 years.8
One of the primary questions of this trial was: Is it a delay of recurrence, or is it an actual cure? Will the survival be longer if you treat them adjuvantly versus waiting until they recur and then treating them for metastatic disease? We don’t know that.
The benefit in terms of DFS was in almost every group. The patients with stage IB disease did not do quite as well. The hazard ratio was 0.39 [95% CI, 0.18-0.76] by investigator assessment. But the DFS hazard ratio in the patients with stage II and IIIA tumors was 0.17 [95% CI, 0.08-0.31] and 0.12 [95% CI, 0.07- 0.20], respectively.9 This is impressive.
What were the CNS recurrence rates in this trial?
We know that osimertinib penetrates the CNS. This was one of the theoretical benefits of this trial—to reduce recurrence in the CNS with osimertinib. At least preliminarily, in the first couple of years, it appears that few patients recur in the CNS on osimertinib versus about 20% of the placebo group who recurred in the CNS.8
[Regarding adverse events (AEs)], there are more in the osimertinib group. Ten percent of patients taking osimertinib had grade 3 AEs, with only 3% having a serious AE. Grade 1 events included diarrhea, which was most common, paronychia, dry skin, pruritus, and stomatitis. Importantly, there were no treatment- related deaths in the study.9
Can you justify giving osimertinib only to a patient who does not want chemotherapy?
We don’t know what the right thing is, but a lot of people don’t want chemotherapy. Part of our job is to convince them that it’s important that they get adjuvant chemotherapy because that will improve their chance of cure. Having these data in hand and seeing how big a magnitude of difference there is in DFS, I know that many more people are going to want to drop the chemotherapy and go right on to adjuvant osimertinib. That is not what this study supports. In this study, it was adjuvant chemotherapy and then going on to the TKI for most of those patients with stage II and III tumors. In my mind, that’s what should be recommended.
About 20% of patients with stage III and about 30% of patients with stage II disease did not get adjuvant chemotherapy in both arms. As already stated, the DFS hazard ratio was a bit more impressive in those who got chemotherapy than in those who didn’t. But it was not that different. I think it would be hard to advise omitting that, especially since we don’t yet know if this cures people.
A good observation that I have not heard many people point out is that progression-free survival in stage IV disease is only about 19 months, whereas, at 36 months, greater than 80% of people were still free of recurrence with about a 50% absolute difference in recurrence rate between the 2 groups. That might suggest that [this therapy] may be more effective in earlier-stage patients. Maybe there are fewer resistant clones at that point, so you get a longer duration of control, or they could be curing people. But either of those potentially could be beneficial or lead to a survival benefit.
References:
1. Kalemkerian GP, Narula N, Kennedy EB, et al. Molecular testing guideline for the selection of patients with lung cancer for treatment with targeted tyrosine kinase inhibitors: American Society of Clinical Oncology endorsement of the College of American Pathologists/International Association for the Study of Lung Cancer/Association for Molecular Pathology Clinical Practice Guideline Update. J Clin Oncol. 2018;36(9):911-919. doi:10.1200/JCO.2017.76.7293
2. Spigel DR. Discussion of LBA5. Presented at: 2020 American Society of Clinical Oncology Virtual Scientific Program; June 1-4, 2020. Accessed November 5, 2020. https://www.bit.ly/38gIEd8
3. Douillard JY, Rosell R, De Lena M, Riggi M, Hurteloup P, Mahe MA; Adjuvant Navelbine International Trialist Association. Impact of postoperative radiation therapy on survival in patients with complete resection and stage I, II, or IIIA non-small-cell lung cancer treated with adjuvant chemotherapy: the adjuvant Navelbine International Trialist Association (ANITA) Randomized Trial. Int J Radiat Oncol Biol Phys. 2008;72(3):695-701. doi:10.1016/j.ijrobp.2008.01.044
4. Le Pechoux C, Pourel N, Barlesi F, et al. An international randomized trial, comparing post-operative conformal radiotherapy (PORT) to no PORT, in patients with completely resected non-small cell lung cancer (NSCLC) and mediastinal N2 involvement: primary end-point analysis of LungART (IFCT-0503, UK NCRI, SAKK) NCT00410683. Ann Oncol. 2020;31(suppl 4):S1178. doi:10.1016/j.annonc.2020.08.2280
5. Pennell NA, Neal JW, Chaft JE, et al. SELECT: a phase II trial of adjuvant erlotinib in patients with resected epidermal growth factor receptor-mutant non-small-cell lung cancer. J Clin Oncol. 2019;37(2):97-104. doi:10.1200/JCO.18.00131
6. Kelly K, Altorki NK, Eberhardt WEE, et al. Adjuvant erlotinib versus placebo in patients with stage IB-IIIA non-small-cell lung cancer (RADIANT): a randomized, double-blind, phase III trial. J Clin Oncol. 2015;33(34):4007-4014. doi:10.1200/JCO.2015.61.8918
7. Zhong WZ, Wang Q, Mao WM, et al; ADJUVANT investigators. Gefitinib versus vinorelbine plus cisplatin as adjuvant treatment for stage II-IIIA (N1-N2) EGFR-mutant NSCLC (ADJUVANT/CTONG1104): a randomised, open-label, phase 3 study. Lancet Oncol. 2018;19(1):139-148. doi:10.1016/S1470-2045(17)30729-5
8. Wu YL, Tsuboi M, He J, et al; ADAURA Investigators. Osimertinib in resected EGFR-mutated non-small-cell lung cancer. N Engl J Med. 2020;383(18):1711-1723. doi:10.1056/NEJMoa2027071
9. Herbst RS, Tsuboi M, Jogn T, et al. Osimertinib as adjuvant therapy in patients (pts) with stage IB-IIIA EGFR mutation positive (EGFRm) NSCLC after complete tumor resection: ADAURA. J Clin Oncol. 2020;38(suppl 18):LBA5. doi:10.1200/JCO.2020.38.18_suppl.LBA5

FDA Clears Lazertinib/Amivantamab for First-Line EGFR-Mutated NSCLC
August 20th 2024Lazertinib and amivantamab as a first-line treatment for patients with locally advanced or metastatic non–small cell lung cancer with specific EGFR mutations demonstrated superior efficacy compared with standard treatment.
Read More
Osimertinib Offers New Standard of Care in Stage III EGFR-Mutated NSCLC
August 6th 2024Suresh S. Ramalingam, MD, discussed the practice-changing findings and implications of the phase 3 LAURA study investigating osimertinib for the treatment of patients with EGFR-mutated non–small cell lung cancer.
Read More