Gertz Assesses Notable Adverse Events of Talquetamab for Relapsed/Refractory Multiple Myeloma
During a Case-Based Roundtable event, Morie Gertz, MD, discussed the safety profile of talquetamab as a single agent and in combination with daratumumab in patients with relapsed/refractory multiple myeloma.
Morie Gertz, MD
Professor of Medicine
Mayo Clinic
Rochester, MN

CASE SUMMARY
- A 63-year-old man who is a part-time consultant to a local law firm was initially diagnosed 8 years ago with multiple myeloma (del[17p]).
Intermediate risk
- International Staging System (ISS) stage II
- He now presents with disease progression post 4 prior lines of therapy that included the following:
- VRd (bortezomib [Velcade], lenalidomide [Revlimid], and dexamethasone) followed by autologous stem cell transplant and lenalidomide maintenance
- DKd (daratumumab [Darzalex], carfilzomib [Kyprolis], and dexamethasone)
- KPd (carfilzomib, pomalidomide [Pomalyst], and dexamethasone)
- Venetoclax (Venclexta) and dexamethasone
- Past medical history including hypertension
- ECOG performance status: 0
- The patient, now 71 years old, presents in follow-up to his oncologist with complaints of increasing bone pain in his left hip and new onset pain in the contralateral hip.
Bone Marrow Biopsy
- 80% λ light chain restricted
- Immunohistochemistry (IHC): B-cell maturation antigen–positive (BCMA+) plasma cells: 63%
Repeat Imaging Study
- PET scan: increased uptake at L4, L5, and bilateral hip
Treatment
- Patient is referred to a chimeric antigen receptor (CAR) T-cell treatment center approximately 30 miles from his home.
- He completes treatment with ciltacabtagene autoleucel (Carvykti).
- Due to his high tumor burden, he remains hospitalized.
- He spends 21 days under observation for therapeutic response and CAR T-cell therapy–related cytokine release syndrome (CRS), neurotoxicity, and tumor lysis syndrome.
- Seventeen Months Later: November 2023
- Patient presents to his oncologist with complaints of increased weakness and dyspnea.
- He remains essentially housebound and no longer drives but is capable of self-care without assistance.
- ECOG performance status: 2
Post–CAR T-Cell Therapy Diagnostic Workup
- Repeat IHC: BCMA+ plasma cells: 63%
- Repeat fluorescence in situ hybridization: acquired gain/amplification (1q) chromosomal abnormality
- Repeat PET scan: bone lesions at L4, L5, and bilateral hips enlarged by 60%
Treatment
- The patient is predosed with oral dexamethasone, diphenhydramine, and acetaminophen.
- Talquetamab (Talvey) is initiated at step-up dosing for 3 days (days 1, 3, and 5).
- Forty-eight hours later, patient begins his weekly treatment dose to be continued until disease progression or unacceptable toxicity.
- He has a grade 1 rash that responds to topical steroids and emollients.
- He has grade 1 dysgeusia and nail changes.
- Over the course of the next 9 months, the patient achieves a very good partial response, which endures into the following year.
Targeted Oncology: How should a patient like this who receives talquetamab be treated for adverse events (AEs)?
GERTZ: The patient is premedicated to try and reduce the reaction. Like all bispecifics, there’s step-up dosing on day[s] 1, 3, and 5, and that’s done to prevent tumor lysis syndrome.1 After the step-up dosing, you can go to full dose once a week, and the way the trials were designed, they were designed to [give it] weekly until progressive disease.
One of the issues with talquetamab is [that] the protein is also found in the skin and in the nails and requires a fair amount of supportive care. This patient developed a rash, which is extremely common, but it was responsive to lipid emollients and topical steroids. They developed taste change— that can be a real problem because we don’t want these patients to lose weight [when] things don’t taste right—and nail changes. But in any case, this patient did achieve a very good partial response.
How did tolerability affect dose modification of talquetamab based on the MonumenTAL-1 trial (NCT03399799) data?
AEs led to dose reduction at the lowest dose in 14.7% [of patients, at the higher dose in] 8.3%, and either dose in 9.8%.2 Discontinuation of therapy occurred in 4.9% with low dose, 8% at the higher dose, and 7.8% with either dose. [The investigators observed] taste changes and the skin and nail toxicities [Table 12,3]. [Three] patients discontinued because of skin problems, and 2 [discontinued because of] dysgeusia.
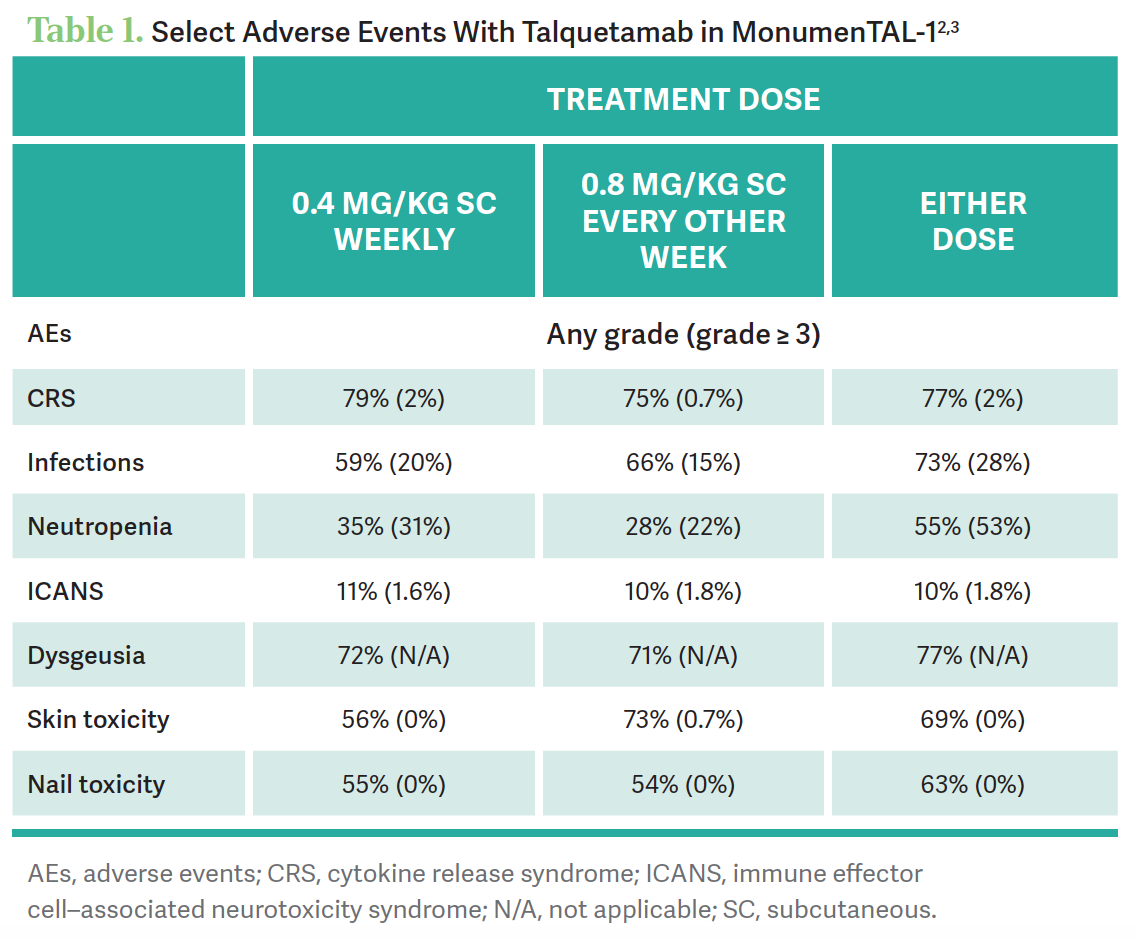
They got an overall response rate of [approximately] 70%. [Since the trial included] patients [with] penta-refractory [disease, and] patients who have had prior BCMA therapies, whether that be bispecific or CAR T-cell therapy, it’s got a place as long as we can control infections and have a reasonable plan to manage the [AEs] associated with the skin toxicity as well as the nail toxicity.
How do academic centers share management of patients who require bispecific therapy?
[At the Mayo Clinic in Rochester, Minnesota], we see a lot where patients are referred to us for bispecific therapy requesting either CAR T-cell therapy or bispecifics, and we take care of them for the first month. Once we’re clear that there isn’t going to be any more CRS or immune effector cell–associated neurotoxicity syndrome [ICANS] and cytopenias are adequately managed, we will return them to the community for the ongoing long-term administration. We are involved in that quite a bit. We share this type of structure where we kick it off just for the initial [cycles], and patients come, [but] they don’t want to stay with us anyway.
What barriers are there to community oncologists introducing bispecific therapies to their practice?
The pharmacy has to be certified, and the provider has to take an online course certifying that [they] know how to manage CRS and ICANS and have some knowledge about when it’s going to occur. I think the certification process is a barrier for a lot of [practices] because a lot of providers are often involved [with each] patient.
What adverse events were observed when talquetamab was combined with daratumumab in the TRIMM-2 trial (NCT04108195)?
[In terms of] the safety profile of talquetamab and daratumumab, the rate of grade 3 or 4 cytopenias, which we all deal with in our practice with chemotherapy, was not particularly high [Table 24]. We know how to deal with neutropenia, thrombocytopenia, and anemia. Those types of hematologic adverse events [were not] an issue, and no unexpected AEs were observed compared with each agent alone. The majority were grade 1 and 2, and talquetamab and daratumumab did not have overlapping toxicities. One patient of 29 discontinued due to an adverse event, [which is] not bad. Cytopenias were seen in the step-up [doses] and the first 2 cycles and resolved in the majority of patients. Neutropenia usually resolved within 1 week.
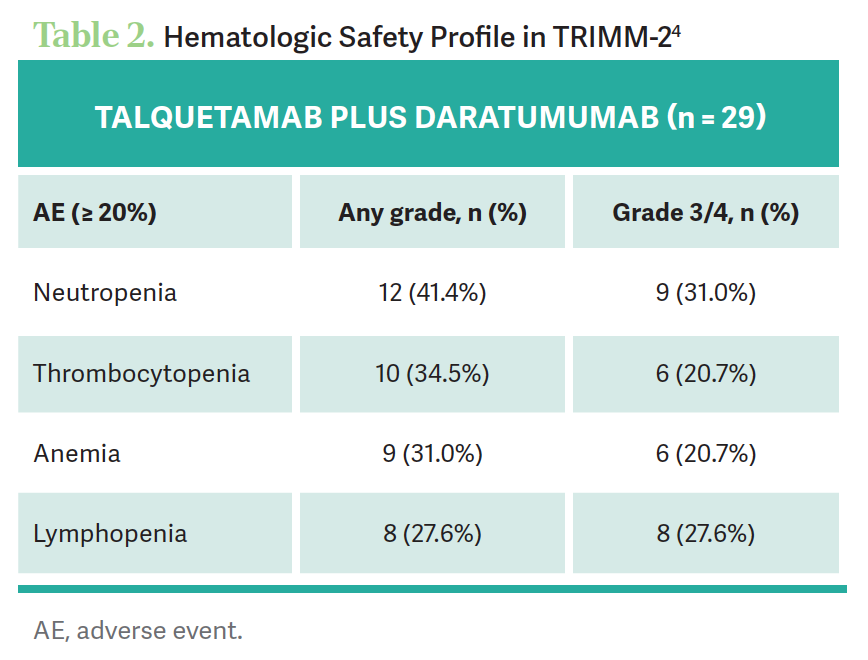
[In terms of] the nonhematologic AEs, the important thing is to look at the oral adverse events, most of which are dysgeusia, and the skin and nail toxicities, which [can be managed] with topical treatments. But notice that grade 3 and 4 skin events [occurred in] 8% [of patients, which is] not trivial. The majority were low grade [83.4% in the higher-dose cohort] but still significant. CRS tended to be only grade 1 and 2; there were no grade 3 or 4 CRS events. Overall, when we look at the grade 3 and 4 toxicities at these various dose levels, [besides] oral and skin AEs, everything else is under 5%. You usually can treat the skin and the nails topically.
Dose reductions were used from 0.8 mg/kg every other week to 0.4 mg/kg every other week [in 16.9% of patients]. Stopping it [was needed] in only 1.5% due to skin toxicity. Usually, topical steroids or oral steroids [can be used], and for the taste changes, mouthwash and saliva [substitute] for the dry mouth. Notice that 77% did have taste alterations, which can cause significant weight loss; 6% required dose reduction. A lot of these patients come in underweight; they’ve had a lot of prior therapy. They’re largely malnourished, and they don’t want to eat anything.
What do you see as the most challenging AE related to bispecific T-cell engagers?
We’ve seen some [severe] infections, [such as] herpes and CMV [cytomegalovirus], that I’m not used to seeing in the pretargeted therapy era. These can occur late, whereas the CRS is usually grade 1 or 2 and is usually done by a month. [But] infections are unpredictable. Although dysgeusia [can lead to] stopping therapy, [infections] scare me because you can’t even do decent surveillance for the patients. They [develop] some exotic viruses.
The problem is we don’t have very good prophylactic antivirals. I’m using acyclovir, I’m using fluconazole, and I’m using a fluoroquinolone, usually levofloxacin. For some of these patients, I’m giving intravenous immunoglobulin. But what are those going to do if someone’s going to get a disseminated CMV? Some of these are [unusual] infections, and I assume it’s because their T cells are [nearly] nonexistent. That worries me going forward. I just think that…giving drugs every other week in patients who have a deep response until progression isn’t sustainable. As we go forward and we try to learn about it, I suspect for maintenance, [we may try] giving a little less frequently. That’s speculative and requires trials. But some of these infections are [challenging], so it’s important to be aware. They’re [also on prophylactic sulfamethoxazole/trimethoprim] because of Pneumocystis pneumonia. They are on a lot of [medications] altogether, and it’s problematic.
If you look at infection rates reported with talquetamab and the BCMA bispecifics, do you see any trends or differences?
We’re talking about small numbers, and that is a dangerous cross-trial comparison. You have to be on guard for any T-cell mediated infections. We’ve done this before with other agents, for antibodies for chronic lymphocytic leukemia that resulted in profound T-cell depletion and all types of exotic, fungal, atypical, mycobacterial infections and the like. I don’t know that one [bispecific] is better than the other, but I don’t think that we’ve got the dosing and the frequency right once patients have achieved their response. It strikes me that once someone gets a response, knowing those infection rates, which can occur quite late, we need to start thinking if we’re not going to continue at the same dose and frequency to progression.
We’re going to start looking at either a dose or frequency reduction…. That doesn’t mean we’re going to reduce infection, because we have no data. That’s something that has to be explored as to whether we’re using too much, because using too much is the typical history for multiple myeloma. We used too much dexamethasone in the beginning, too much thalidomide in the beginning, too much bortezomib, carfilzomib, and belantamab mafodotin [Blenrep]. If we were a little smarter with belantamab, I doubt we would have had all the eye [toxicities]. These maximum tolerated doses are probably not the minimum effective dose. We ought to figure out better ways. But I don’t think that there’s an infection advantage that I can discern yet among bispecifics.
REFERENCES
1. Talvey. Prescribing information. Janssen Pharmaceutical Companies; 2023. Accessed January 30, 2023. https://tinyurl.com/4tczj285
2. Touzeau C, Schinke C, Minnema M, et al. Pivotal phase 2 MonumenTAL-1 results of talquetamab (tal), a GPRC5DXCD3 bispecific antibody (BSAB), for relapsed/refractory multiple myeloma (RRMM). Hemasphere. 2023;7(S3):e5955094. doi:10.1097/01.HS9.0000967676.59550.94
3. Chari A, Minnema MC, Berdeja JG, et al. Talquetamab, a T-cell-redirecting GPRC5D bispecific antibody for multiple myeloma. N Engl J Med. 2022;387(24):2232-2244. doi:10.1056/NEJMoa2204591
4. Dholaria BR, Weisel K, Mateos MV, et al. Talquetamab (tal) + daratumumab (dara) in patients (pts) with relapsed/refractory multiple myeloma (RRMM): updated TRIMM-2 results. J Clin Oncol. 2023;41(suppl 16):8003. doi:10.1200/JCO.2023.41.16_suppl.8003

Advances in Subsequent Therapies Shake Up Sequencing of ccRCC Treatment
April 25th 2024With the approval of belzutifan and other newer data for treating patients with recurrent renal cell carcinoma, the state of subsequent therapies is advancing beyond the reuse of frontline options with impacts on duration of response and quality of life.
Read More
Age, Disease Burden Are Factors in Early Use of Selinexor in Multiple Myeloma
April 22nd 2024During a Case-Based Roundtable® event, Jonathan L. Kaufman, MD, discussed treatment approaches and the tolerability of a selinexor-containing regimen in a patient with relapsed/refractory multiple myeloma in the first article of a 2-part series.
Read More
Novel Approaches Focus on Limiting Toxicity in Older Patients With ALL
April 22nd 2024The major challenges for clinicians treating older patients with acute lymphoblastic leukemia surround the emergence of resistance to existing therapies and the toxicities associated with current chemotherapies.
Read More