George Discusses Potency of VEGF TKIs in Advanced Renal Cell Carcinoma
During a Case-Based Roundtable® event, Saby George, MD, discussed the case of a patient with clear cell renal cell carcinoma who progressed after left nephrectomy followed by treatment with axitinib plus pembrolizumab at recurrence.
Saby George, MD, FACP
Associate Professor
Jacobs School of Medicine and Biomedical Sciences
University at Buffalo
Professor of Oncology and Medicine
Director of Network Clinical Trials
Department of Medicine
Roswell Park Comprehensive Cancer Center
Buffalo, NY

CASE SUMMARY
A 71-year-old male patient with a history of metastatic renal cell carcinoma (mRCC), status post left nephrectomy and adrenalectomy, was diagnosed with clear cell RCC (ccRCC) with an adrenal metastasis.
4 Years Later
- Disease recurrence: lung nodules on biopsy consistent with ccRCC
- Recognized that nodules had been present on scans during the previous 2 years.
- Based on low volume and indolent behavior of disease as well as patient preference, he was observed.
18 Months Later
- Follow-up scans indicate continued indolent tumor growth, increased total tumor burden, new paratracheal lymph nodes (2.0 × 1.5 cm), and multiple growing pulmonary nodules.
- A decision was made to initiate systemic therapy with 200 mg of pembrolizumab (Keytruda) given intravenously once every 3 weeks plus 5 mg of axitinib (Inlyta) twice a day.
Patient Follow-Up
- Stable disease
- He denied fever or sick contact.
- Influenza vaccine was up to date.
- He reported moderate diarrhea, well controlled with antidiarrheal medication, and mild fatigue.
Post-Treatment Cycle 6
- The patient developed progressive fatigue, mild dyspnea, and mild cough without chest pain.
- Pulse oximetry was 93% at rest.
- Infectious disease workup: negative for bacterial, fungal, or viral organisms
- Chest CT at approximately 18 weeks: confirmed grade 3 pneumonitis
Treatment
- Oral prednisone at 50 mg was prescribed once a day for 4 weeks followed by a taper down to 10 mg once a week every 4 weeks.
- Pembrolizumab was discontinued, while axitinib was continued.
14 Months After Initiating Systemic Therapy
- The patient reported increasing back pain, mild nausea, weight loss, and new onset of persistent rib pain.
- Imaging confirmed disease progression:
- Growth of paratracheal lymph nodes (was 20 × 15 mm; now 25 × 28 mm)
- New mediastinal and hilar nodal involvement
- New retroperitoneal lymph nodes
- New lytic osseous lesions
- ECOG performance score: 1
Please describe the available therapies for a patient case like this.
GEORGE: The VEGF tyrosine kinase inhibitors [TKIs] basically target the blood vessel. Immune checkpoint inhibitors are useful when there is [resistance], which can be overcome by using checkpoint inhibition.1 [Looking at the] profile of various kinds of VEGF TKIs, the first-generation drugs like sunitinib [Sutent] and sorafenib [Nexavar] are not as potent as the latest drugs, like axitinib and tivozanib [Fotivda]. The third-generation TKIs are potent, and that’s why we use a lower dose of those drugs…compared [with] the other TKIs. There are various other TKIs that inhibit VEGF, along with other targets, [including pazopanib (Votrient), cabozantinib (Cabometyx), and lenvatinib (Lenvima)].1 All the TKIs…hit the vascular site of [their targets]; the belzutifan, temsirolimus, everolimus hit the cancer [cells]; and the immune checkpoint inhibitors, like CTLA-4 and PD-1/PD-L1 inhibitors, block the signaling pathways.2
What is the risk factor for a patient like this and the preferred treatment?
All the factors [in a patient case like this]…put the patient into a favorable-risk group. [According to the National Comprehensive Cancer Network guidelines,] the preferred treatment regimens in the latest version for the favorable-risk group of patients include either the axitinib/ pembrolizumab combination, cabozantinib/nivolumab combination, or the lenvatinib and pembrolizumab combination.3 All of them are category 1 recommendations, and there are other options, but I won’t talk about them in this case.3
What were the efficacy results that favor tivozanib?
The TIVO-3 trial [NCT02627963] led to the approval of tivozanib in this setting.4 [It included patients with advanced ccRCC] who had failed 2 or 3 prior regimens, so this is third- or fourth-line therapy, including VEGF TKIs.5 Patient stratification was done [based on their] prior regimen and [International mRCC Database Consortium] criteria. They were then [randomly assigned] in a 1:1 fashion to receive either tivozanib or sorafenib. Tivozanib was dosed at 1.5 mg on [a] 3-weeks-on, 1-week-off regimen. Sorafenib was given at 400 mg twice a day continuously in a 4-week cycle. Treatment [was given] until disease progression or unacceptable toxicity. The primary end point was progression-free survival [PFS], which was assessed by blinded independent central review. Secondary end points included overall survival [OS], objective response rate [ORR], durable objective response [DOR], and safety. The…vast majority of patients were in the intermediate-risk grouping, and the favorable- and poor-risk groupings [had a similar number of patients enrolled]. The majority of the patients were mostly from the European Union and North America. The prior treatment regimen was well balanced and included patients either on a TKI/PD-1, TKI-TKI, [or] TKI and other drug combination.5
What was the PFS with this therapy?
The median PFS was 5.6 months vs 3.9 months favoring tivozanib with [an] HR of 0.73.6 There was a consistent 27% reduction of risk of disease progression or death on tivozanib. When you look at the 2-year PFS rates, you could see that it’s 18% vs 5% favoring tivozanib.6 In another landmark analysis done for long-term PFS in the intention-to-treat population…12.3% of patients were progression free at the 3-year mark and 7.6% at the 4-year mark on tivozanib opposed to 2.4% and 0%, respectively, on the sorafenib arm.7 So, that’s pretty impressive in the third line with patients doing that well [Table7]. It’s not quite the plateauing of the curve, but it looks like it when you look at the patients who went beyond the 2-year mark.7 PFS per [independent review committee] in the patients who had prior immunotherapy showed that the PFS rate at 2 years was 25% with tivozanib, meaning if patients had a prior immunotherapy they did not progress while on this drug, which is pretty impressive.7
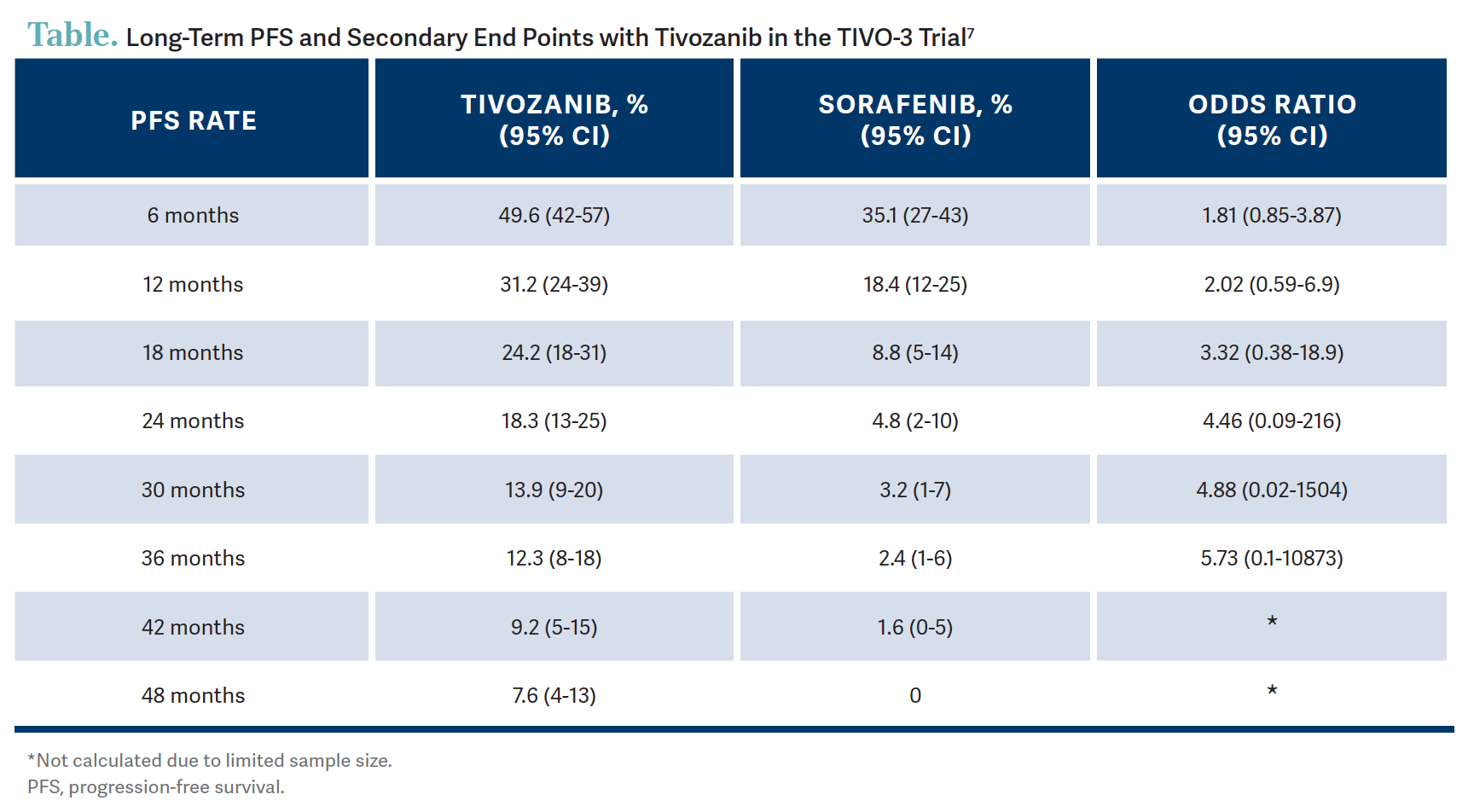
What were the secondary end point results?
The ORR was 23% for patients on tivozanib vs 11% for sorafenib, and the disease control rates were 82% and 69%, respectively. DOR with tivozanib was 20.3 months compared with 9.0 months on sorafenib, again favoring the tivozanib arm.7 With the OS the HR dropped over time. In August 2019 the OS HR was 0.99 [95% CI, 0.76-1.29], then in 2020 the HR was 0.97 [95% CI, 0.75-1.24], then it dropped to 0.91 [95% CI, 0.72-1.17] in 2021, and ended at 0.89 [95% CI, 0.70-1.41] in 2021.8 So, it seems like it’s still continuing to mature, and this is a good [outcome]. We see with some drugs that have a long-term efficacy and durable benefits, and this is seen with a lot of checkpoint inhibitors as well.
How should physicians consider toxicities with these therapies?
The practical considerations of using tivozanib importantly include managing diarrhea, nausea, and vomiting without interruption and dose reduction. If dose modifications are required, the dose can be reduced [from 1.34 mg] to 0.89 mg.4 Again, the full doses are 1.34 mg daily on a 3-weeks-on, 1-week-off regimen, and 0.89 mg is the next lower dose level. It is also recommended, as I mentioned, for a 3-weeks-on, 1-week-off cycle, and administration [of the therapy] can be with or without food. Adverse event management is paramount, as with any other TKI, and diarrhea, nausea, vomiting, and [similar adverse events] take precedence in making sure that the patients stay on the drug at the most optimal dose, so that they get the best benefit [of their treatment].4
REFERENCES
1. Bukavina L, Bensalah K, Bray F, et al. Epidemiology of renal cell carcinoma: 2022 update. Eur Urol. 2022;82(5):529-542. doi:10.1016/j. eururo.2022.08.019
2. Barragan-Carrillio R, Govindarajan A, Rock A, Sperandio RC, Pal SK. Managing metastatic renal cell carcinoma after progression on immunotherapy. Hematol Oncol Clin North Am. 2023;37(5):965-976. doi:10.1016/j.hoc.2023.05.005
3. NCCN. Clinical Practice Guidelines in Oncology. Kidney cancer, version 2.2024. Accessed February 2, 2024. http://tinyurl.com/cyv7n8kr
4. FDA approves tivozanib for relapsed or refractory advanced renal cell carcinoma. FDA. March 10, 2021. Accessed February 2, 2024. http://tinyurl.com/ mtd6pt2m
5. Rini BI, Pal SK, Escudier BJ, et al. Tivozanib versus sorafenib in patients with advanced renal cell carcinoma (TIVO-3): a phase 3, multicentre, randomised, controlled, open-label study. Lancet Oncol. 2020;21(1):95-104. doi:10.1016/S1470-2045(19)30735-1
6. Rini BI, Pal SK, Escudier B, et al. TIVO-3: a phase III, randomized, controlled, multicenter, open-label study to compare tivozanib to sorafenib in subjects with refractory advanced renal cell carcinoma (RCC). Data presented at: 2019 Genitourinary Cancers Symposium; February 14-16, 2019; San Francisco, CA. Abstract 541.
7. Atkins MB, Verzoni E, Escudier B, et al. Long-term PFS from TIVO-3: tivozanib (TIVO) versus sorafenib (SOR) in relapsed/refractory (R/R) advanced RCC. J Clin Oncol. 2022;40(suppl 6):362. doi:10.1200/ JCO.2022.40.6_suppl.362
8. Rini BI, Pal SK, Escudier B, et al. Maturation of overall survival (OS) in TIVO-3 with long-term follow-up. J Clin Oncol. 2022;40(suppl 16):4557. doi:10.1200/ JCO.2022.40.16_suppl.4557

Advances in Subsequent Therapies Shake Up Sequencing of ccRCC Treatment
April 25th 2024With the approval of belzutifan and other newer data for treating patients with recurrent renal cell carcinoma, the state of subsequent therapies is advancing beyond the reuse of frontline options with impacts on duration of response and quality of life.
Read More
Enhancing Precision in Immunotherapy: CD8 PET-Avidity in RCC
March 1st 2024In this episode of Emerging Experts, Peter Zang, MD, highlights research on baseline CD8 lymph node avidity with 89-Zr-crefmirlimab for the treatment of patients with metastatic renal cell carcinoma and response to immunotherapy.
Listen
Beyond the First-Line: Economides on Advancing Therapies in RCC
February 1st 2024In our 4th episode of Emerging Experts, Minas P. Economides, MD, unveils the challenges and opportunities for renal cell carcinoma treatment, focusing on the lack of therapies available in the second-line setting.
Listen
Ornstein Advises on Starting Dose and Management of Lenvatinib in RCC
April 21st 2024During a Case-Based Roundtable® event, Moshe Ornstein, MD, MA, provided guidance on dosing and toxicity concerns in a patient treated with lenvatinib plus pembrolizumab for advanced renal cell carcinoma.
Read More