Rizzieri Considers Prognostic Models and Trial Data for Myelofibrosis
During a case-based roundtable event, David A. Rizzieri, MD, discussed the case of a 68-year-old patient with myelofibrosis.

David A. Rizzieri, MD, a medical oncologist at Novant Health Cancer Institute in Charlotte, NC, discussed the case of a 68-year-old patient with myelofibrosis during a Targeted Oncology case-based roundtable event.
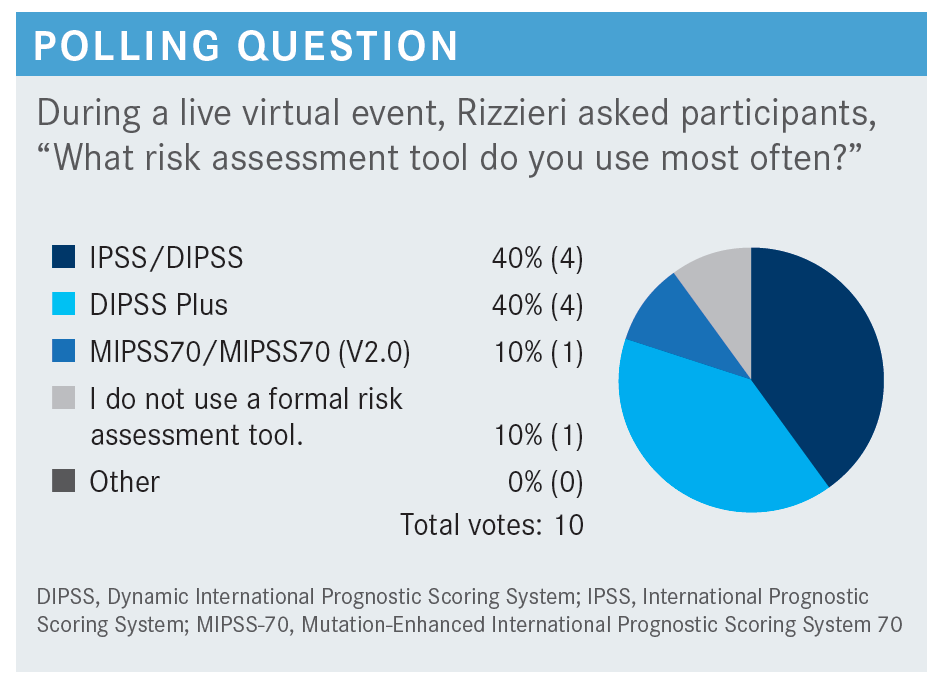
RIZZIERI: The [prognostic models of MF] can differentiate the anticipated survival, which helps us understand when we might intervene with ruxolitinib [Jakafi], other approved therapies, or even transplant. These [models have undergone] evolution over time as we’ve understood more about the disease—from IPSS [International Prognostic Scoring System] to DIPSS [Dynamic IPSS] to DIPSS Plus.
The initial IPSS used easy, objective data. Currently, we like to add to that the karyotypic information when available.1,2 A lot of the patients with MF may not have that, but in patients [from whom] we can get this information, it improves prognostic abilities. There is also improvement with adding transfusion dependence, platelet count, etc.
The other aspect with DIPSS vs IPSS is that a time-dependent variable is built into DIPSS. Technically, IPSS would only be used at the time of diagnosis, but DIPSS can be used throughout the course of the patient’s illness. Whatever the patient’s score is when they’re seeing you in the office that day, you look at their survival from that point forward, not from the previous initial diagnosis. Prognostically, [this] is helpful when we’re thinking about other interventions.
The DIPSS and DIPPS Plus can help us in the low-, intermediate-, and high-risk groups to differentiate anticipated survival.3 The intermediate-2 and high-risk patients are those I would offer something potentially curative, such as allogeneic [transplant], if they’re a candidate and accepting of the risks. For those with lower risk, I would observe or treat them with other measures, depending on the circumstances.
How does the MIPSS70 (Mutation-Enhanced IPSS 70) help assess patients?
The MIPSS70 is really gaining ground. Of course, now we’re using MIPSS70-plus and 2.0 versions. Tefferi et al4 had a very similar, although slightly different, scale. The MIPSS70 had objective measures, such as counts in peripheral blasts, just like the DIPSS had. But then there is more importance to getting a bone marrow [test] and looking at the degree of fibrosis, [as well as] the genetic analysis for the absence of a calreticulin type 1 [CALR] mutation]. The [high-molecular risk] categories are those with highly mutated regions. Specifically in the MIPSS70, they are ASXL1, EZH2, SRSF2, and IDH1/2.4,5
Fluorescence in situ hybridization or molecular genetics can be helpful, because when you use this prognostic score, having 1 of these mutations is important in the ranking. If you have 2 or more, you have a higher risk ranking. The MIPSS70-plus adds in additional genetic information. This type of information is helpful because it differentiates the low-risk patients [who have a median overall survival (OS) of 2.7 years vs the high-risk patients who have a median OS of 2.3 years].6
So, we can have an important discussion with the patient on where they fall in terms of expected changes in the illness and what that might mean for when we [would] intervene with potential therapies. Our patient is high risk and they had at least 5 points [based on only bone marrow analysis], so I’m quite concerned about them progressing sooner rather than later, based on this model.
The MIPSS70-plus 2.0 incorporates further very high– risk karyotypes, and the U2AF1 Q157 mutation status, as well as sex, and defines 5 prognostic categories.6 Again, the very low-risk and low-risk patients do well, and depending on symptoms, we would look at those patients differently than the very high-risk and high-risk patients, [for whom] we may intervene differently.
How do other prognostic models and guidelines suggest managing a patient such as this?
There’s another available calculator for us to be aware of, which I find helpful—the MYSEC-PM [Myelofibrosis Secondary to PV and ET Prognostic Model]. This is a prognostic model specifically for patients with newly diagnosed post-PV [polycythemia vera] MF or post-ET [essential thrombocythemia] MF.7 These were the patients I saw a lot in a referral pattern when I was at Duke University Hospital doing transplants. Specialists in hematologic malignancies may see a skewed version of these patients, because after being ill for a long time, they get sent to [those] specialists. We typically would lump these patients as having secondary MF and being high risk, but that’s not the way they all are based on the MYSEC-PM.
Passamonti et al7 published a paper a few years ago, where they dissected high-risk and low-risk patients based on easy-to-obtain criteria, such as the standard objective hemogram blast, the genotype, and constitutional symptoms. The scores differentiated low-risk and high-risk groups. So in a patient with post-PV MF or post-ET MF, if they’re low risk, that’s a different conversation than the one about the high-risk or intermediate–2 risk patient who clearly has a different survival pattern. For our case patient, unfortunately, if they had post-PV or post-ET MF, they would have had a higher risk status, if that were the clinical scenario we were dealing with here.
The NCCN [National Comprehensive Cancer Network] guidelines are easily available to many of us.8 I find their guidance helpful. It doesn’t specify a specific right way to do things, because there’s more than 1 way to [treat]. The important thing is it gives us the arena we should be working in, and if we’re outside that arena, we’re probably doing something that’s not up-to-date. But if you’re within the NCCN guidelines, there’s an appropriate amount of variability, depending on your own practice pattern. So the MIPSS70, the DIPSS Plus, or the regular DIPSS can all be appropriately applied and can be reasonable in differentiating lower and higher risk. The stratum in those models can be helpful.
What therapy would you recommend for this patient?
Both ruxolitinib and fedratinib [Inrebic] are fair choices. She’s 68 years old, but if she has a robust [biological age] with nonablative therapy, certainly a referral for transplant to discuss risk and benefits is not unreasonable. But a majority of patients with MF don’t end up getting transplant for a variety of reasons.
Based on the NCCN guidelines, this patient would fall into the category of platelets more than 50 × 109/L. [She is] not a transplant candidate, which then leads to the 2 approved options [ruxolitinib and fedratinib]. The other options are if she was not a transplant candidate and had symptomatic anemia, which I don’t think she had, vs if she was a transplant candidate, [which], in that case, she would go for an allogeneic transplant consult.8
Being a transplant candidate is tough. It depends on who’s seeing the patient. So if it’s unclear, it’s certainly worth a call to your transplant colleague to ask and at least have a consultation for many of these patients.
Which data support the use of ruxolitinib for MF in the frontline setting?
The data that informed some of the NCCN guidelines were based on the phase 3 COMFORT-I [NCT00952289] and COMFORT-II [NCT00934544] trials for ruxolitinib. COMFORT-I was a double-blind, placebo-controlled study for MF, with a 1:1 randomization. COMFORT-II used the best available therapy (BAT) instead of placebo, with a 2:1 randomization.9,10
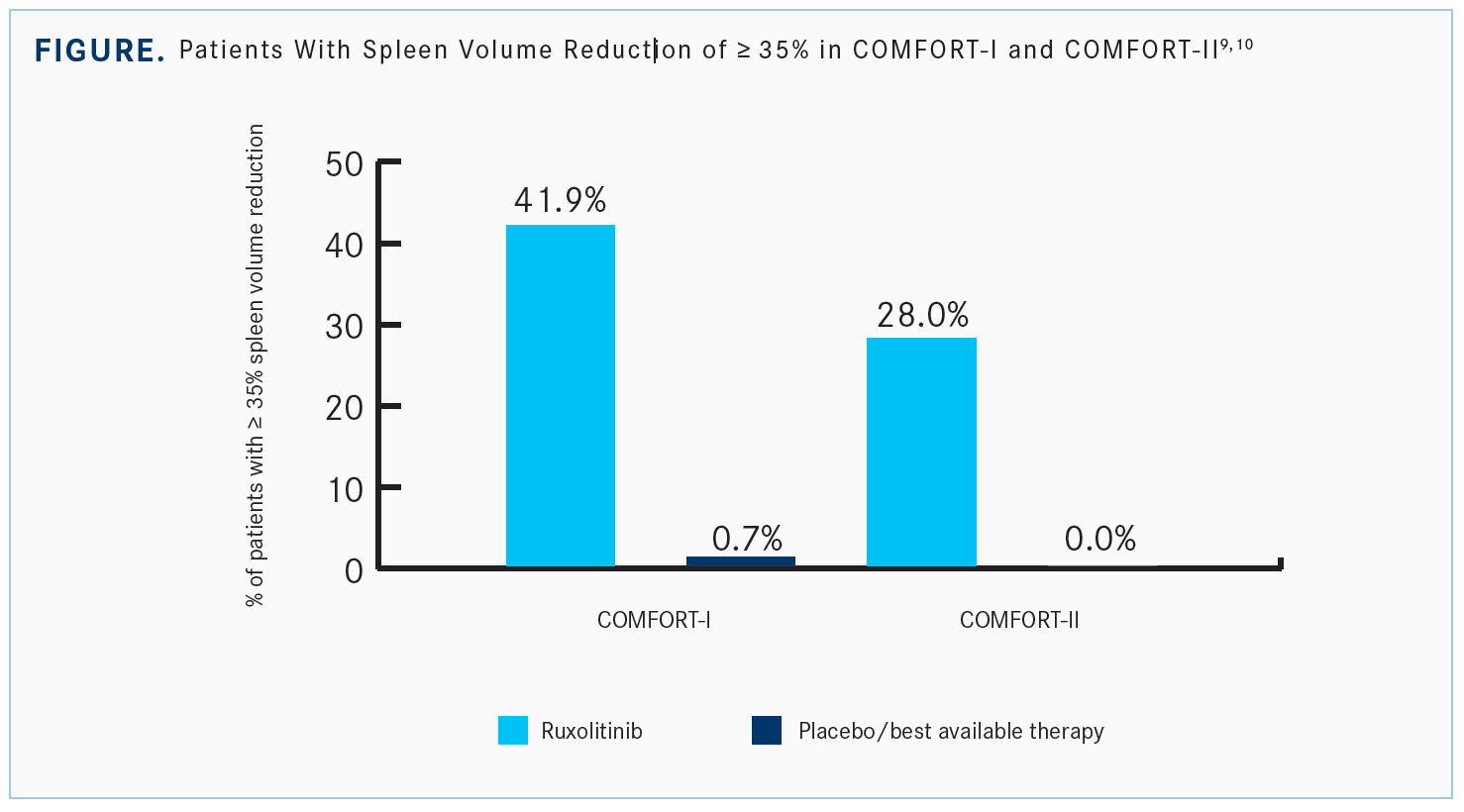
In both trials, patients had at least a 35% reduction in the spleen volume [Figure9,10]. Many of these patients present with a large spleen, which is related to many of the other issues. This difference was important.
In COMFORT-I, 1 patient on placebo got a decrease in spleen volume because of an infarct, [which is] not what we like to see for our patients. But there’s a large portion of patients on ruxolitinib who overcame that 35% decrease. Even in those who didn’t meet the 35% decrease, a high proportion had some decrease in the splenic volume.9
Again, the splenic volume in COMFORT-II showed a significant decrease, with a higher proportion receiving at least a 35% reduction. There were very few whose spleen size increased while on ruxolitinib.10
COMFORT-I analyzed symptom response, which is a big aspect for a lot of our patients. We have the numbers and the physical exam findings, but how do the patients feel? They did exploratory analyses with the results published for the longer-term follow-up results.9,10
There was a significant symptom reduction for ruxolitinib. What’s important is what specifically got better clinically. Abdominal discomfort and pain—of course, [because of reduced] splenomegaly—early satiety, night sweats, pruritus, muscle pains, and others in the symptom analysis trended to improvement with ruxolitinib. The COMFORT-II symptom response is a very similar story.9,10
How did patients do, in terms of efficacy end points, in the COMFORT trials?
The OS data for the 2 studies were pooled, as they were not set up initially to look at that. There was crossover over time, which impacts survival, etc. But, with the benefit of time and following over 1000 patients, retrospectively, the COMFORT-I and COMFORT-II intent-to-treat groups had a median survival of [approximately] 70 months [95% CI, 61-76].11
The data suggest there may be an improvement with ruxolitinib therapy, which is interesting because for many of these patients, it was not ruxolitinib vs nothing, it was ruxolitinib vs the ability to cross over. So that’s an interesting thought. Some [final causes of death at final analysis] for patients were transformational leukemia, progression of MF, thrombosis, bleeding, infection, etc.11
The splenic response was also noted to correlate with outcomes of ruxolitinib treatment. So the OS by spleen response at 6 months. Importantly, the durability of splenic response correlated with OS.12
The efficacy by titrated dose from COMFORT-I is important when we’re working together with some of our colleagues on the phone, as we mentioned we don’t see this very often. It’s difficult to safely choose the right dose for many of these patients, as 25 mg twice a day may be too much for some. You don’t want to undertreat. We know [that for many patients], 5 mg twice a day is just not enough, but we don’t want to scare them away with treatment, either. So start with something low, make sure they tolerate it, get their trust that they’re not going to get too sick from it, and titrate up.
The key is starting low at 10 mg twice a day and escalating quickly to a maximum safe dose, because you’re going to know in a couple months whether the therapy works. You don’t want to undertreat at 5 mg twice a day for those 2 months. You [have] to be at a dose that can be effective, so at least 10 mg twice a day, titrating up appropriately to 15 mg, 20 mg, 25 mg. The splenic volume and total symptom score clearly improve in response when you’re at 10 mg twice a day or higher.13
Hematologic grade 3 or 4 AEs [adverse events] include cytopenia, which is probably going to be worse than if they were on BAT or placebo. [Approximately] half thepatients had worsening cytopenia, particularly anemia.9,10
Which other studies have looked at ruxolitinib in this patient population?
[The EXPAND trial (NCT01317875)] had 2 strata based on platelet counts of patients: those with platelets between 75 and 99 × 109/L [n = 44] and those with platelets between 50 and 74 × 109/L [n = 25]. They started at 5 mg twice a day and escalated based on the cohorts that were proven to be safe. Both groups came to a recommended dosing of 10 mg twice a day to start, then escalate based on tolerance.14,15
The EXPAND study final analysis looked at thrombocytopenia [and anemia in both groups]. The data suggest cytopenia are going to be common—something we must manage through.15 In patients with platelet counts of 50 to 100 × 109/L—even though we were starting at 10 mg twice a day and escalating up—if you can keep these patients on therapy, they still have a very high chance of a splenic response. Then with that, the chance in symptom score in both strata, as well.14
Which data support the use of fedratinib for MF in the frontline setting?
The JAKARTA study [NCT01437787] is the study that primarily led to the approval of fedratinib in patients with intermediate-2 or high-risk MF.16 There are lots of data now supporting this approved agent. The randomization was 1:1 for fedratinib at 400 mg or 500 mg a day vs placebo, and 96 patients were randomized to each arm.17
The response by platelet count was interesting. [The group with platelet counts of under 100 × 109/L had a spleen response of 36%, and those with over 100 × 109/L had a response of 49%. I don’t know [whether] this is a clinically significant number in this small group of patients. There is a trend, but again, I don’t know that it’s real. It was similar with symptom response, with those with platelet counts under 100 × 109/L having a response of 31%, and those with over 100 × 109/L platelets having a response of 42%.17
There are AEs to be aware of, particularly the GI [gastrointestinal] ones with fedratinib. Approximately 50% to two-thirds of patients had some GI effects with fedratinib, but the grade 3 and 4 GI AEs were not so significant. The anemia was 30% for grade 3 and 4 in the fedratinib group and 10% in the placebo group. In addition, the laboratory abnormalities, including hemogram changes, were in both groups, but maybe slightly more accentuated in fedratinib compared with placebo in this regard.18
The one concern that might be different in the JAKARTA study was the black box warning that came out of the data for the serious and fatal encephalopathy, including Wernicke encephalopathy and encephalopathy of unclear origin.19
REFERENCES
1. Bose P, Verstovsek S. The evolution and clinical relevance of prognostic classification systems in myelofibrosis. Cancer. 2016;122(5):681-692. doi:10.1002/ cncr.29842
2. Cervantes F, Dupriez B, Pereira A, et al. New prognostic scoring system for primary myelofibrosis based on a study of the International Working Group for Myelofibrosis Research and Treatment. Blood. 2009;113(13):2895-2901. doi:10.1182/blood-2008-07-170449
3. Gangat N, Caramazza D, Vaidya R, et al. DIPSS plus: a refined Dynamic
International Prognostic Scoring System for primary myelofibrosis that incorporates prognostic information from karyotype, platelet count, and transfusion status. J Clin Oncol. 2011;29(4):392-397. doi:10.1200/JCO.2010.32.2446
4. Tefferi A, Nicolosi M, Mudireddy M, et al. Revised cytogenetic risk stratification in primary myelofibrosis: analysis based on 1002 informative patients. Leukemia. 2018;32(5):1189-1199. doi:10.1038/s41375-018-0018-z
5. Guglielmelli P, Lasho TL, Rotunno G, et al. MIPSS70: Mutation-Enhanced International Prognostic Score System for transplantation-age patients with primary myelofibrosis. J Clin Oncol. 2018;36(4):310-318. doi:10.1200/JCO.2017.76.4886
6. Tefferi A, Guglielmelli P, Lasho TL, et al. MIPSS70+ Version 2.0: Mutation and Karyotype-Enhanced International Prognostic Scoring System for primary myelofibrosis. J Clin Oncol. 2018;36(17):1769-1770. doi:10.1200/JCO.2018.78.9867
7. Passamonti F, Giorgino T, Mora B, et al. A clinical-molecular prognostic model to predict survival in patients with post polycythemia vera and post essential thrombocythemia myelofibrosis. Leukemia. 2017;31(12):2726-2731. doi:10.1038/ leu.2017.169
8. NCCN. Clinical Practice Guidelines in Oncology. Myeloproliferative neoplasms, version 1.2022. February 28, 2022. Accessed June 14, 2022. https://bit. ly/2Wcczfa
9. Verstovsek S, Mesa RA, Gotlib J, et al. A double-blind, placebo-controlled trial of ruxolitinib for myelofibrosis. N Engl J Med. 2012;366(9):799-807. doi:10.1056/ NEJMoa1110557
10. Harrison C, Kiladjian JJ, Al-Ali HK, et al. JAK inhibition with ruxolitinib versus best available therapy for myelofibrosis. N Engl J Med. 2012;366(9):787-798. doi:10.1056/NEJMoa1110556
11. Verstovsek S, Gotlib J, Mesa RA, et al. Long-term survival in patients treated with ruxolitinib for myelofibrosis: COMFORT-I and -II pooled analyses. J Hematol Oncol. 2017;10(1):156. doi:10.1186/s13045-017-0527-7
12. Palandri F, Palumbo GA, Bonifacio M, et al. Baseline factors associated with response to ruxolitinib: an independent study on 408 patients with myelofibrosis. Oncotarget. 2017;8(45):79073-79086. doi:10.18632/oncotarget.18674
13. Verstovsek S, Gotlib J, Gupta V, et al. Management of cytopenias in patients with myelofibrosis treated with ruxolitinib and effect of dose modifications on efficacy outcomes. Onco Targets Ther. 2013;7:13-21. doi:10.2147/OTT.S53348
14. Vannucchi AM, Te Boekhorst PAW, Harrison CN, et al. EXPAND, a dose-finding study of ruxolitinib in patients with myelofibrosis and low platelet counts: 48-week follow-up analysis. Haematologica. 2019;104(5):947-954. doi:10.3324/ haematol.2018.204602
15. Guglielmelli P, Kiladjian J-J, Vannucchi AM, et al. The final analysis of Expand: a phase 1b, open-label, dose-finding study of ruxolitinib (RUX) in patients (pts) with myelofibrosis (MF) and low platelet (PLT) count (50 × 109/L to < 100 × 109/L) at baseline. Blood. 2020;136(suppl 1):4-5. doi.org/10.1182/blood-2020-137742
16. FDA approves fedratinib for myelofibrosis. FDA. Updated August 16, 2019. Accessed June 14, 2022. https://bit.ly/3dErcBr
17. Pardanani A, Harrison C, Cortes JE, et al. Safety and efficacy of fedratinib in patients with primary or secondary myelofibrosis: a randomized clinical trial. JAMA Oncol. 2015;1(5):643-651. doi:10.1001/jamaoncol.2015.1590
18. Pardanani A, Tefferi A, Masszi T, et al. Updated results of the placebo-controlled, phase III JAKARTA trial of fedratinib in patients with intermediate-2 or high-risk myelofibrosis. Br J Haematol. 2021;195(2):244-248. doi:10.1111/bjh.17727
19. Inrebic. Prescribing information. Impact Biomedicines Inc; 2019. Accessed June 14, 2022. https://bit.ly/33FJ9Zx

Connecting Spleen Volume Reduction to Survival Outcomes in MF
April 21st 2024During a Case-Based Roundtable® event, Raajit K. Rampal, MD, PhD, discussed the correlation between spleen volume responses and survival outcomes for patients with myelofibrosis in the second article of a 2-part series.
Read More
Savona Discusses First-Line JAK Inhibition for Patients With Myelofibrosis at Risk of Anemia
April 17th 2024During a Case-Based Roundtable® event, Michael Savona, MD, and participants discussed the case of a patient with myelofibrosis and moderate anemia receiving JAK inhibitor therapy.
Read More
PTCy Offers New Hope for Mismatched Stem Cell Transplants in Leukemia, MDS
April 13th 2024Jeff Auletta, MD, discussed how PTCy-based graft-vs-host disease prophylaxis offers a promising approach for expanding access to successful cell transplantation regardless of donor match or patient ethnicity.
Read More