RMC-6236 Shows Safety, Efficacy in KRAS G12X Tumors
RMC-6236 was safe and tolerable in patients with mutant solid tumors harboring KRAS G12X mutations across various cancer types.
Alexander Spira MD, PhD, FACP

The investigational oral treatment RMC-6236 appeared safe and tolerable, alongside a reduction in tumor growth for patients with mutant solid tumors harboring KRAS G12X mutations across various cancer types. Investigators conducted a phase 1/1b multicenter open-label study trial (NCT05379985) to assess the dosage, safety, and efficacy of RMC-6236, according to an American Association for Cancer Research (AACR) news release.1,2
“Overall, 27 of 54 patients (50%) were evaluable for change in mutant KRAS VAF on treatment. RMC-6236 demonstrated a well-tolerated safety profile across dose levels and in patients with diverse tumor types. The treatment also demonstrated dose-dependent pharmacokinetics compatible with once-daily dosing and achieved exposures predicted to induce tumor regressions, Alexander Spira MD, PhD, FACP, said in a presentation at the 2023 AACR-NCI-EORTC International Conference on Molecular Targets and Cancer Therapeutics.3 Spira is a clinical assistant professor at Johns Hopkins School of Medicine in Baltimore, Maryland; and a medical oncologist and codirector of the Thoracic and Phase I Program at Virginia Cancer Specialists Research Institute in Fairfax.
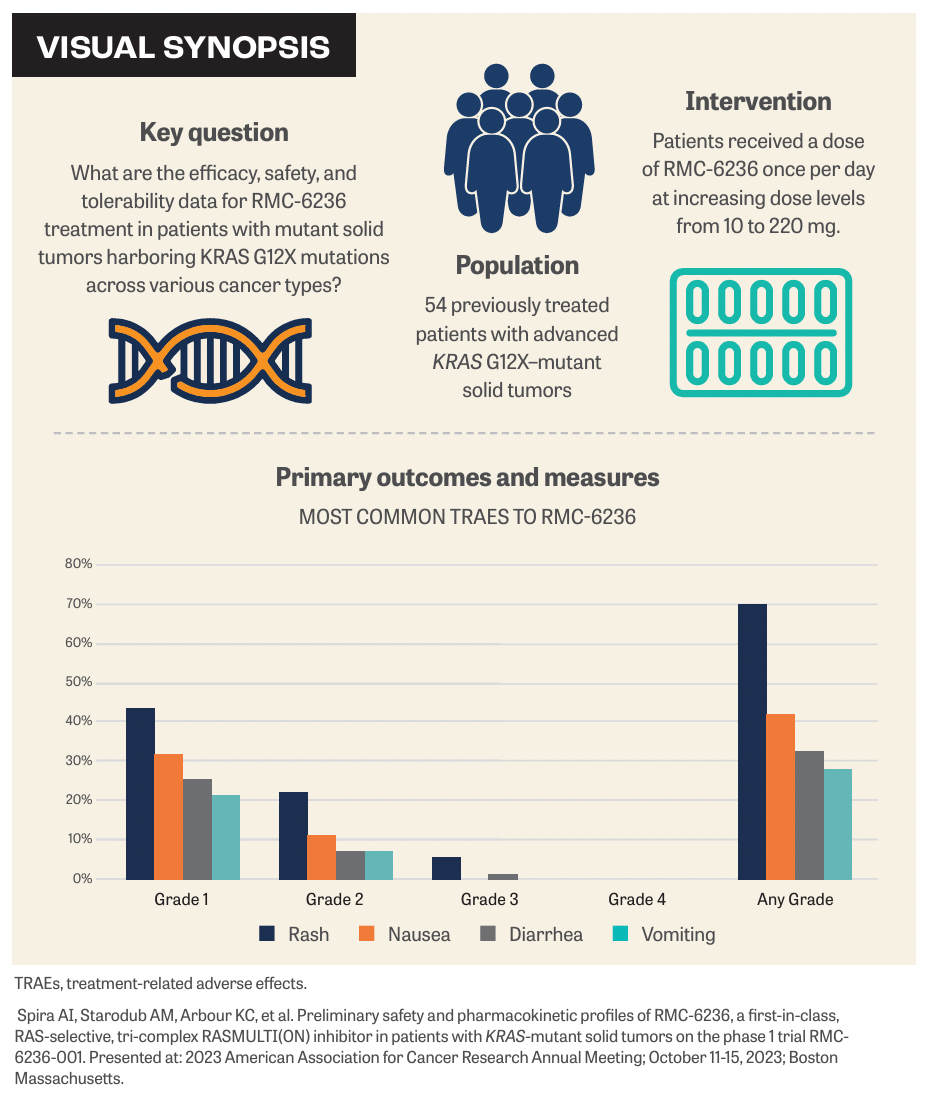
The total study population of 131 patients received RMC-6236 at increasing dose levels of 10 to 220 mg once per day beginning on May 11, 2023. The median age was 64 years (range, 30-86) and the patient population was mostly men (53%). Patients had advanced KRAS G12X mutant solid tumors in various cancer types: pancreatic cancer (n = 26; 48%), non–small cell lung cancer (n = 13; 24%), and colorectal cancer (n = 10; 19%).2
The most common treatment-related adverse events (TRAEs) were rash (acneiform [35%] or maculopapular [13%]) and gastrointestinal- related toxicities (nausea [32%], diarrhea [19%], vomiting [15%]) and were grade 1 or 2 in severity. These TRAEs occurred in 10% of patients and rash frequency, excluding severity, was proportional to the dose. One patient reduced their dose because of grade 2 acneiform rash.
The emergence of an acneiform or maculopapular rash aligns with the intended effect of RAS pathway inhibitors. This rash typically manifested in cycle 1 or 2 and was predominantly of grade 1 or 2 intensity. Supportive measures included the use of topical antibiotics, topical steroids, and/or oral antibiotics.3
Among patients with pancreatic cancer, 1 had grade 3 pancreatitis and 1 developed a perforation in the large intestine where the tumor was situated, necessitating dose discontinuation. Nonetheless, the tumor did exhibit a reduction in size following treatment.
Twenty-three patients (43%) discontinued treatment for various reasons. Among them, 17 patients (31%) did so due to progressive disease per RECIST v1.1, 2 clinical progression, 1 had a perforated large intestine (previously mentioned), 1 was removed based on investigators’ decision, 1 died, and 1 patient withdrew from the trial.1
To address the need for treatments that target KRAS mutations beyond G12C, RMC-6236 targets the active form of RAS proteins, not just KRAS G12C, leading to potentially deeper and more lasting regression of tumors. RMC-6236 has the potential to be effective against a wider range of cancer types due to its ability to target various RAS mutations as well as target wild-type KRAS (the nonmutated form) and KRAS with mutations at other positions.1
RMC-6236 exhibited dose-dependent increases in exposure without any notable accumulation in the blood after repeated daily dosing and at a dose of 220 mg given once daily. Furthermore, promising early indications of antitumor activity, including partial responses as per RECIST v1.1 criteria, were noted across various tumor types and dosage levels. Additionally, molecular responses were observed, characterized by a reduction in circulating tumor DNA associated with the KRAS-mutated allele and other somatic mutations, aligning with the anticipated inhibition of mutant RAS by RMC-6236, which allows for the treatment of a range of tumors.1
The oral administration of RMC-6236 demonstrates favorable pharmacokinetics and overall tolerability particularly at doses that lead to tumor reduction, according to the news release. However, dose escalation is ongoing and the optimal dosage for the next phase of clinical trials remains to be determined.1
“There are a lot of people who did not think you could do a RAS (ON) inhibitor, surmising it would be too toxic because you are turning off normal RAS. We found out that you can, and be okay, which was very surprising. More steps need to be taken going forward, but we might be able to target a lot of different mutations right now, which is a first. The key takeaways are that we can do this, it’s active, and we're very excited,” Spira told Targeted Therapies in Oncology in an interview.
REFERENCES
1. Investigational inhibitor of active RAS shows clinical promise. News release. American Association for Cancer Research. Accessed October 12, 2023. https://aacr.ent.box.com/s/qp3go91uhgye1tfc98tcr48e71agn5l5
2. Evaluation of RMC-6236 in subjects with advanced solid tumors harboring specifi c mutations in KRAS. Clinicaltrials. gov. Updated December 27, 2022. Accessed October 12, 2023. https://clinicaltrials.gov/study/NCT05379985
3. Spira AI, Starodub AM, Arbour KC, et al. Preliminary safety and pharmacokinetic profi les of RMC-6236, a first-in-class, RAS-selective, tri-complex RASMULTI(ON) inhibitor in patients with KRAS-mutant solid tumors on the phase 1 trial RMC6236-001. Presented at: 2023 American Association for Cancer ResearchAnnual Meeting; October 11-15, 2023; Boston Massachusetts.

Overcoming Barriers in Cancer Clinical Trials: A Path Forward for Better Patient Care
April 29th 2024Clinical trials play a pivotal role in developing effective therapies, yet their integration is challenged by issues such as insufficient reimbursement structures, misaligned incentives, physician burnout, and a complex regulatory environment.
Read More
Gholam Analyzes Treatment Outcomes for Advanced HCC in Child-Pugh B Population
April 28th 2024During a live Community Case Forum event in partnership with the Tennessee Oncology Practice Society, Pierre Gholam, MD, examined the current state of treatment for patients with hepatocellular carcinoma, looking in particular at what data is available for those with Child-Pugh B and C status who have poorer outcomes and have limited data from prospective clinical trials.
Read More