Epcoritamab Demonstrates Antitumor Activity in R/R CLL
Findings from the EPCORE CLL-1 trial were presented by Arnon Kater, MD, PhD, during the 2023 International Workshop on CLL, held October 6 to 9, 2023, in Boston, Massachusetts.
Arnon Kater, MD, PhD
Full Professor of Clinical
Haematology, All-Cancer
Immunology, and Cancer Biology and Immunology
Amsterdam University Medical Centers
Amsterdam, The Netherlands

Epcortimab-bysp (Epkinly), as a single agent, demonstrated promising antitumor activity in high-risk patients with relapsed/refractory chronic lymphocytic leukemia (R/R CLL) in the expansion cohort of the phase 1b/2 EPCORE CLL-1 trial (NCT04623541). Findings were presented by Arnon Kater, MD, PhD, during the 2023 International Workshop on CLL, held October 6 to 9, 2023, in Boston, Massachusetts.1
“Today I present the data of the expansion cohort of 23 patients,” Kater, full professor of clinical haematology, all-cancer immunology, and cancer biology and immunology at Amsterdam University Medical Centers in the Netherlands, said during the presentation.
Responses were seen early, frequently, and appeared durable, with approximately 83% of responders remaining in response at 9 months. “The objective response rate [ORR] was 82% with a complete response [CR] rate of 33%, which I thought was quite encouraging, especially in difficult-to-treat, high-risk patients with relapsed and refractory CLL,” Kater said. The median time to response was 1.9 months (range, 1.6-3.7) and the median time to CR was 3.6 months (range, 1.6-10.8). At 9 months, the estimated duration of response, progression-free survival, and overall survival was 83%, 67%, and 81%, respectively.
To be included in the trial, patients had to have CD20-positive R/R CLL; have received 2 or more prior lines of systemic therapy, including treatment with or intolerance to a Bruton tyrosine kinase (BTK) inhibitor; and have an ECOG performance status of 0, 1, or 2.
In the dose-expansion cohort (N = 23), patients received subcutaneous epcoritamab at the recommended phase 2 dose of 48 mg. Patients received epcoritamab with step-up dosing, with a 0.16 priming dose and 0.8 mg intermediate doses before the first full dose. Corticosteroid prophylaxis was provided to mitigate cytokine release syndrome (CRS).
Patients underwent efficacy assessment by CT/ MRI obtained every 8 weeks through cycle 6, and at 24 weeks thereafter. Treatment continued until disease progression. The primary end point was ORR and secondary end points included CR rate, time to response, and safety/tolerability.
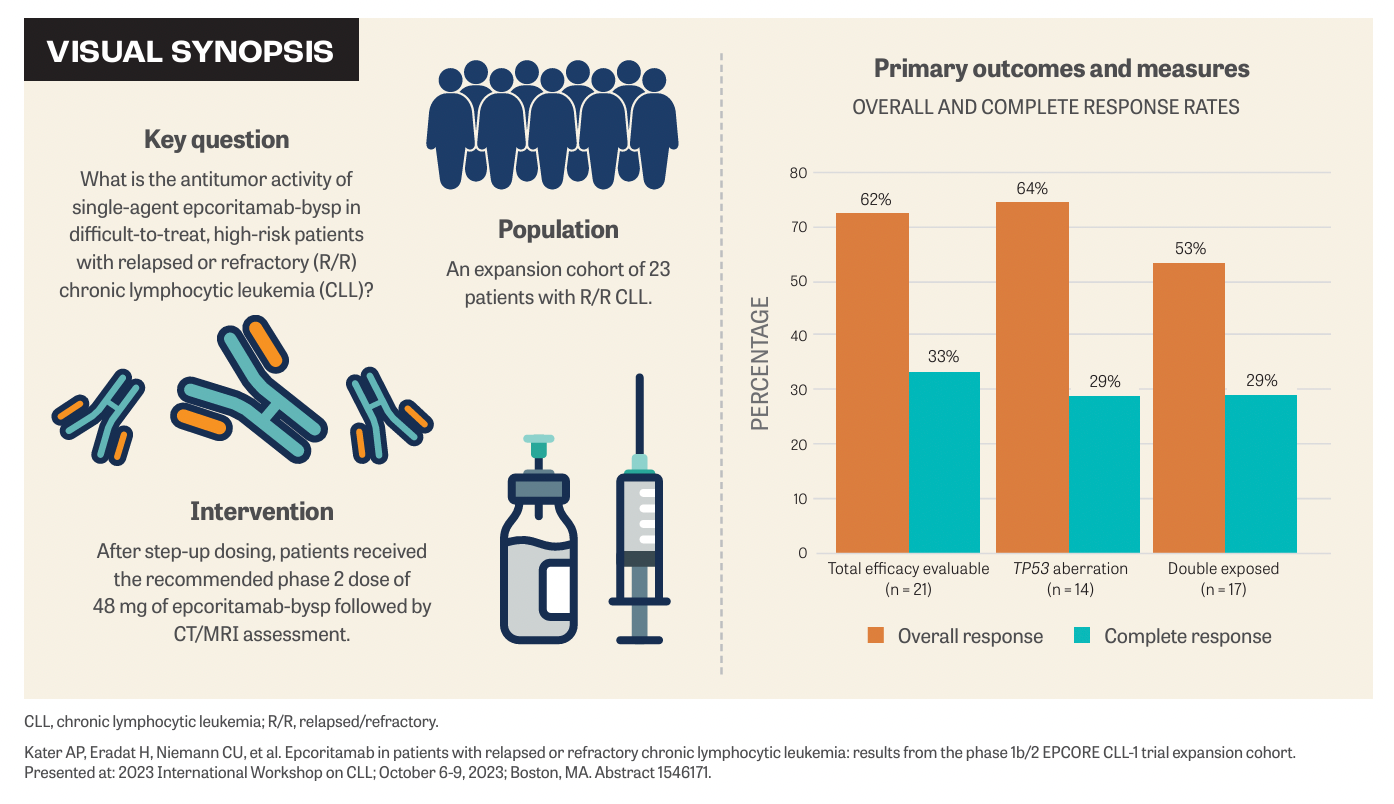
The median age of patients was 72 years (range, 55-83) and most (74%) were men. Seventy percent of patients were IGHV mutated and 65% had a TP53 aberration. In this heavily pretreated population, the median number of prior lines of therapy was 4 (range, 2-10) and 65% had undergone 4 or more prior lines of therapy.
All patients were treated with small molecules and BTK inhibitors and 74% discontinued BTK inhibitor therapy because of disease progression. Eighty-three percent had been treated with a BCL-2 inhibitor and 4% had undergone chimeric antigen receptor T-cell therapy.

The most common treatment-emergent adverse events (TEAEs) were CRS, thrombocytopenia, and anemia. “Most patients entered the study with preexisting cytopenias,” Kater said. TEAEs were primarily grade 1 to 2, and 2 TEAEs led to treatment discontinuation. There were 3 fatal TEAEs reported, 1 of which was pneumonia and was treatment- related. “There were no CLL progressions among the responders; however, there were 3 cases of Richter transformation,” Kater said. After deeper analysis, Kater said, “1 of those 3 patients was found to have a T-prolymphocytic leukemia in the marrow prior to the study.”
CRS resolved in all patients, according to Kater. The median time to onset after full dose was 7.3 hours (range, 1-99) and median time to resolution was 3 days (range, 1-16).
“Importantly, all immune [effector] cell-associated neurotoxicity syndrome [ICANS] events occurred very early in the context of grade 2 CRS disease,” Kater said. “One patient had clinical tumor lysis syndrome, which was grade 2, and no case of ICANS or tumor lysis led to discontinuation,” Kater added.
Epcoritamab is a novel subcutaneous CD20/CD3 bispecific antibody that offers patients who have been previously treated with BTK and BCL-2 inhibitors another treatment option.
Previous trials from EPCOR CLL-1 show encouraging efficacy and manageable safety in R/R CLL (dose escalation) and Richter syndrome (dose expansion).2
Overall, Kater said CRS occurrence was predictable, with most cases following the first full dose. No AEs of special interest led to discontinuation, with all cases resolving. He encouraged continued exploration of the agent combined with other therapies, noting that patients with R/R CLL/small lymphocytic lymphoma or Richter syndrome were currently being enrolled in trials.
REFERENCES
1. Kater AP, Eradat H, Niemann CU, et al. Epcoritamab in patients with relapsed or refractory chronic lymphocytic leukemia: results from the phase 1b/2 EPCORE CLL-1 trial expansion cohort. Presented at: 2023 International Workshop on CLL; October 6-9, 2023; Boston, MA. Abstract 1546171.
2. Kater AP, Ye JC, Sandoval-Sus J, et al. Subcutaneous epcoritamab in patients with Richter’s syndrome: early results from phase 1b/2 trial (EPCORE CLL-1). Blood. 2022;140(suppl 1):850-851. doi:10.1182/blood-2022-158298

Advances in Subsequent Therapies Shake Up Sequencing of ccRCC Treatment
April 25th 2024With the approval of belzutifan and other newer data for treating patients with recurrent renal cell carcinoma, the state of subsequent therapies is advancing beyond the reuse of frontline options with impacts on duration of response and quality of life.
Read More
Ornstein Advises on Starting Dose and Management of Lenvatinib in RCC
April 21st 2024During a Case-Based Roundtable® event, Moshe Ornstein, MD, MA, provided guidance on dosing and toxicity concerns in a patient treated with lenvatinib plus pembrolizumab for advanced renal cell carcinoma.
Read More