Roundtable Discussion: Gadgeel Discusses Challenges in Testing and Treating Patients With Non–Driver-Mutated Metastatic NSCLC
A 59-year-old Asian man presented with chest pain, cough, and dyspnea. A discussion of the case was led by Shirish M. Gadgeel, MD, during a Targeted Oncology Case-Based Roundtable event.
Shirish M. Gadgeel, MD


During a Targeted Oncology Case-Based Roundtable, Shirish M. Gadgeel, MD, division head of the Division of Hematology and Oncology and associate director of Patient Experience and Clinical Care at Henry Ford Health System in Detroit, MI, discussed the case of a 59-year-old Asian man with metastatic non–small cell lung cancer.
GADGEEL: At this point, what further testing do you do? Do you do only tissue-based testing? Do you do liquid- and tissue-based testing, or only liquid-based testing?
SABAGH: I would do EGFR, ALK, and ROS1 testing. Where I practice, they do the BRAF locally, so they can get those results quickly. I would add EGFR, ALK, ROS1, and BRAF on the specimen.
GADGEEL: Do you always send your tissue locally? Or do you also send it outside?
SABAGH: If any of those come back positive, then that’s all I need at that point. If they come back negative, I have the option for chemotherapy plus immunotherapy vs immunotherapy only, depending on the PD-L1. I rule out any of those mutations before I decide on adding immunotherapy.
WASELENKO: I prefer next-generation sequencing [NGS] on the tumor itself, if possible, because then we’re going to pick up some unusual mutations. And now we have so many inhibitors. For example, that will also tell us if there’s a ROS1 mutation, a MET mutation, NTRK mutation—some of the less common mutations, which may be attractive biologic targets.
KARAMLOU: I usually use liquid biopsy because you get a turnaround result time that’s quick. In that situation, you can get your EGFR/ALK results relatively quickly. I also send tissue to confirm the liquid biopsy results, but I routinely do liquid biopsy up front just because of the rapidity and the fact that you don’t have to hunt for tissue. It just makes it a lot easier.
GADGEEL: Do you do both liquid and tissue in all patients?
KARAMLOU: If I have tissue available, yes, I try to.
CHOWDHARY: It’s good to talk about this as a counterpoint. Because liquid biopsies give you a quick turnaround time, should they be done in all patients? At Cleveland Clinic, our approach is not to do liquid biopsies in everyone. We usually have enough tissue where we do the lung hotspot testing. We do the lung NGS panel. It may take 10 to 14 days, but usually we have enough tissue that we get an adequate result.
We’re not routinely using liquid biopsy unless we really need a quick answer, or if the path[ologist] is from outside and it is going to take us a while to get their NGS panel and so forth. So by and large, we are not using the liquid biopsy in everyone, but there are many at the clinic who do. I want to know what you guys are doing and why. Do you do a liquid biopsy in everyone up front? Or is that something [you decide] case by case?
GADGEEL: I do use liquid biopsy in most, if not all, of my patients. About 50% of the patients I see are from another institution, and I’m never sure how much tissue they have. Even if the patient has had a biopsy here at our institution, sometimes it’s hard to know. The most frustrating thing is when, about a week into the testing, you learn there’s not adequate tissue. One important thing about liquid biopsy: It is helpful when it is positive. If it is positive for one of the targetable alterations, you can use that result and start therapy. If it is negative, you still need to wait because about 30% of the time there could be a targetable alteration in the tumor, but it is missed in the circulating tumor DNA.
However, the concern here is 2-fold. One is that if you look at the targeted therapy results, the response rates are clearly higher. The other concern is that—at least with some targeted agents, maybe not with all— there is increased toxicity when the targeted agent is administered after immunotherapy, particularly if it is administered within a few weeks of the last dose of immunotherapy. Finally, if you look at the targeted therapy results, in many of the targeted agents, the efficacy is better in the front line compared with the recurrent disease setting. For all those factors, even beyond EGFR and ALK for the approved agents, the preference is to use targeted agents first, then use chemotherapy-immunotherapy or immunotherapy.
I would also add that PD-L1 can be somewhat misleading. You can have EGFR and ALK tumors that have high PD-L1 expression. As was the case in this patient, the PD-L1 result comes back relatively early and there can be a tendency, if the PD-L1 is high, to immediately start the patient on pembrolizumab [Keytruda]. There are 2 issues with that. One is there are at least preclinical data to suggest that high PD-L1 expression in EGFR and ALK tumors may not be a result of actual presence of immune cells within the tumor microenvironment.
PD-L1 expression can be a downstream effect of EGFR and ALK signaling. The PD-L1 may be high irrespective of whether there are immune cells in the tumor microenvironment. PD-L1 expression in these targetable non–small cell lung cancers [NSCLCs] may not have the same relevance as PD-L1 expression in other NSCLCs. The other issue, as I mentioned, is there is some concern about using these targeted agents after immunotherapy. So it’s quite critical, as much as possible, to wait for the molecular analysis.
SABAGH: Do you mean the PD-L1 could be a false positive?
GADGEEL: No—it is high. The efficacy of single-agent pembrolizumab or atezolizumab [Tecentriq] or cemiplimab [Libtayo] is higher in high PD-L1 tumors. The reason is that usually—not always—high PD-L1 expression is a result of CD8 T cells present in the tumor microenvironment.
So the immune system is ready to attack the cancer, but in response to the CD8 cells being present in the tumor microenvironment, the tumor upregulates PD-L1 expression and resists the immune attack from the CD8 T cells. When that is the reason why PD-L1 is high, pembrolizumab blocks PD-L1 and the CD8 T cells can attack the cancer cells.
However, in EGFR and ALK, that may happen, but it’s more likely [a downstream effect]. In an EGFR-mutated tumor, the EGFR is mutated, so EGFR is signaling at a higher rate than normal tissue. Similarly, in ALK, the ALK receptor is signaling downstream at a higher rate than normal tissue. One of the effects of that downstream signaling is an increase in PD-L1. It’s not that the PD-L1 is falsely high, but the reason [PD-L1 is high] is not because there are already immune cells, and the tumor is resisting those immune cells; it’s because of downstream signaling for EGFR and ALK. If there are no CD8 immune cells in the tumor microenvironment, then no immune attack can be launched against the tumor, even if you block PD-L1 with pembrolizumab.
I am saying this because when the PD-L1 results come in, there is a tendency to act on it before the molecular results come, but the PD-L1 result is quite critical, as viewed in the context of molecular results. I would also say that, though we must test all tumors in all patients irrespective of their smoking history, I am a lot more patient in waiting for the molecular results in someone like this patient, who had only a 10-pack-year smoking history and had Asian ethnicity. So there’s a little higher likelihood of EGFR mutation. What I’m suggesting is that it is quite important to wait for molecular results.
Now there are clearly situations in NSCLC where the patient is sick, you need to start therapy, and you can’t wait for the molecular results. In that situation, it is prudent to start the patient on chemotherapy alone and wait for the molecular results before initiating immunotherapy, so that you’re not caught in a situation where you started immunotherapy and you find out the patient has a targetable alteration, and you have to switch to targeted therapy.

WINTER: I’m a radiation oncologist. What matters most to us is, if we’re going to provide consolidative immunotherapy—most often in the transition from first to second line—then we’re most comfortable radiating along with an agent we’ve got some experience with.
KNAPP: It’s a little hard to compare across all these different trials. Certainly, I’m looking at [the patient’s] PD-L1 level and their functional status. I do shy away from the combination immunotherapy treatments just because of the adverse events, so I’m more looking at the single immunotherapies. Out of habit, I’m more used to pembrolizumab than atezolizumab [Tecentriq] or nivolumab [Opdivo], so I’ve been using it the most.
GADGEEL: In most cases where PD-L1 is high, do you end up choosing immunotherapy alone or chemotherapy-immunotherapy?
KNAPP: It depends on the patient. I’m usually using immunotherapy alone, unless there’s a patient I feel I need to get a rapid response in, then I might do chemotherapy with immunotherapy.
KUMAR: I do use chemotherapy-immunotherapy, so carboplatin [Paraplatin] and pemetrexed [Alimta, Pemfexy] for the adenocarcinoma, and pembrolizumab or carboplatin, paclitaxel, and pembrolizumab for the squamous.
GADGEEL: Can anybody comment on the clinical scenarios where you’re using nivolumab-ipilimumab [Yervoy] in CheckMate 227 [NCT02477826]?1
KARAMLOU: You have these patients who just don’t want to get chemotherapy. That’s the subgroup of patients I would probably entertain using nivolumab-ipilimumab. I haven’t tried it in the less than 1% [subgroup] because of the label. But more than 1%, if they don’t want to get chemotherapy, I certainly will offer that to them.
GADGEEL: Absolutely. Do you also offer it for patients with high PD-L1? Or do you stick to single agent?
KARAMLOU: No. [If the patient’s] PD-L1 expression is over 50%, I would say 99% of the time I do pembrolizumab alone.
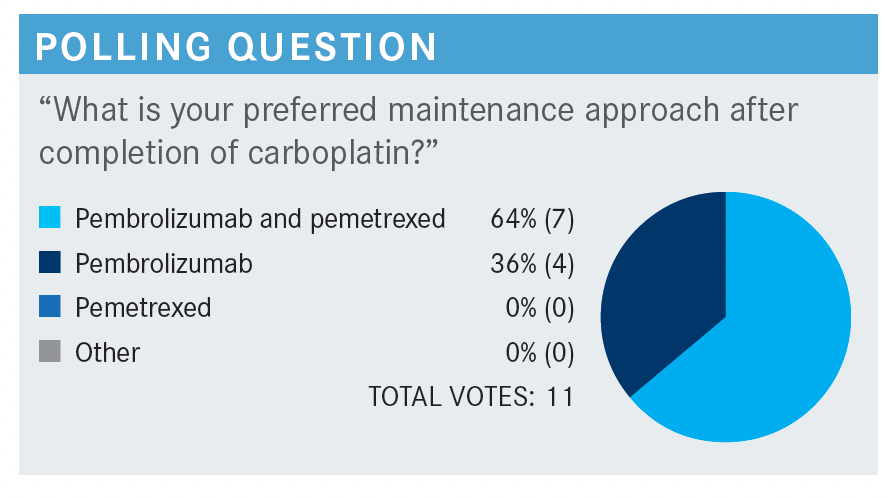
GADGEEL: In KEYNOTE-189 [NCT02578680], pemetrexed plus pembrolizumab could be continued for 2 years.2 At that point, pembrolizumab or placebo was discontinued. Investigators were allowed to continue pemetrexed even beyond 2 years. I want to tell you that the median duration of treatment was approximately [10 months (SD, 7.8 months)] in this study with the combination of pembrolizumab and chemotherapy. Finally, there are a lot of real-world data that suggest many practitioners are discontinuing pemetrexed after 4 cycles and just continuing pembrolizumab.3 In the trial, pemetrexed was continued. We don’t have a clear answer as to whether both drugs need to be continued or not. I generally do pembrolizumab and pemetrexed if the patient can tolerate them. There are some patients who have a challenge tolerating both agents. I may drop 1 of the agents, or sometimes I’ve even changed the schedule to every 4 weeks to improve tolerability, though that was not done in the trial.2

SABAGH: Dr Karamlou mentioned that, for patients with PD-L1 expression greater than 1%, he used the combined immunotherapy, but if [PD-L1 expression] is more than 50%, he uses pembrolizumab. Isn’t more than 1% also extended to more than 50%? Why, then, if the PD-L1 goes to more than 50%, would you go to pembrolizumab and not stick with the immunotherapy-immunotherapy, even though it’s in the same range?
KARAMLOU: I just don’t think that, in that situation, the CTLA-4 adds much, except potentially more toxicity.
GADGEEL: KEYNOTE-598 [NCT03302234], [which] has been published in the Journal of Clinical Oncology, looked at the combination of pembrolizumab plus ipilimumab vs pembrolizumab alone in patients with PD-L1 greater than or equal to 50%.4 That is the only prospective study of immunotherapy combination vs immunotherapy alone in the high PD-L1 population. In that study, there was no survival advantage. As Dr Karamlou mentioned, there was greater toxicity with the combination. We don’t have clear evidence that immunotherapy combination is necessary in the high PD-L1 NSCLC population.

GADGEEL: Have you encountered a situation where there is oligoprogression and your medical oncology colleagues requested radiating the area of oligoprogression, so they can continue the frontline chemotherapy-immunotherapy or immunotherapy?
WINTER: We do encounter this situation. Typically, the things that we’re interested in knowing are: How long has the patient been on their systemic therapy? To what extent is their disease controlled? How many progressive sites do they have? When it comes to consolidating oligometastatic or oligoprogressive disease, [most of our data are] in the setting of 5 lesions or fewer. If we have a relatively limited number of lesions that are progressive on systemic therapy that’s otherwise well tolerated, and we can safely target them with high-dose radiotherapy, we’ll typically offer ablative radiotherapy and try to consolidate all the progressive sites to stall an agent change. But what to do with that agent change, of course, is up to the discretion of the medical oncologist.
GADGEEL: If the patient’s disease progresses after 2 cycles, or maybe even after 4 cycles, and even if the progression appears to be limited to a couple sites, I’m much more inclined to switch that patient’s systemic therapy rather than send the patient for radiation to the progressing sites and continue the frontline therapy. I suspect, in that patient, whose disease has progressed relatively quickly, other sites are going to progress soon as well. I have considered that approach when patients have had disease control, at least for some period. I have an arbitrary cutoff of at least 6 months, but it’s not a hard-and-fast cutoff.
REFERENCES:
1. Ramalingam SS, Ciuleanu TE, Pluzanski A, et al. Nivolumab + ipilimumab versus platinum-doublet chemotherapy as first-line treatment for advanced non-small cell lung cancer: three-year update from CheckMate 227 part 1. J Clin Oncol. 2020;38(suppl 15):9500. doi:10.1200/JCO.2020.38.15_suppl.9500
2. Gadgeel S, Rodríguez-Abreu D, Speranza G, et al. Updated analysis from KEYNOTE-189: pembrolizumab or placebo plus pemetrexed and platinum for previously untreated metastatic nonsquamous non-small-cell lung cancer. J Clin Oncol. 2020;38(14):1505-1517. doi:10.1200/JCO.19.03136
3. Velcheti V, Hu X, Piperdi B, Burke T. Real-world outcomes of first-line pembrolizumab plus pemetrexed-carboplatin for metastatic nonsquamous NSCLC at US oncology practices. Sci Rep. 2021;11(1):9222. doi:10.1038/s41598-021-88453-8
4. Boyer M, Şendur MAN, Rodríguez-Abreu D, et al. Pembrolizumab plus ipilimumab or placebo for metastatic non-small-cell lung cancer with PD-L1 tumor proportion score ≥ 50%: randomized, double-blind phase III KEYNOTE-598 study. J Clin Oncol. 2021;39(21):2327-2338. doi:10.1200/JCO.20.03579

Bispecific Antibodies and ADCs Deliver a Futuristic Horizon Across Lung Cancer Settings
October 23rd 2024Recent advancements in protein engineering, especially antibody-drug conjugates, show promise in lung cancer treatment, with ivonescimab outperforming pembrolizumab in PD-L1-positive advanced non-small cell lung cancer.
Read More