Salit Discusses the Use of Staging and Grading for Patients With GVHD to Choose Appropriate Treatment
Rachel B. Salit, MD, discussed the case of a 48-year-old patient with graft-versus-host-disease.
Rachel B. Salit, MD

Rachel B. Salit, MD, associate professor, Clinical Research Division, Fred Hutchinson Cancer Research Center at the University of Washington School of Medicine in Seattle, WA, discussed the case of a 48-year-old patient with graft-versus-host-disease.

Targeted OncologyTM: What are your thoughts on the currently accepted options for acute GVHD (aGVHD) prophylaxis?
SALIT: Between calcineurin inhibitors, if we have a choice, my preference is usually tacrolimus. Tacrolimus is better tolerated [than cyclosporin] in terms of adverse events [AEs], blood pressure, kidney function, and [even] the smell.
Methotrexate is a tried-and-true prophylaxis, especially in the myeloablative or high-intensity transplant setting. [In contrast], mycophenolate mofetil [MMF]; [CellCept] is usually used in the nonmyeloablative or reduced-intensity setting. When calcineurin inhibitors were used with MMF as prophylaxis for GVHD, the GVHD was higher. That’s why we [use] methotrexate [instead of MMF].1
Sirolimus [Rapamune] is often combined with a calcineurin inhibitor and MMF, or with a calcineurin inhibitor and methotrexate. Sirolimus is very well tolerated, except for some triglyceride AEs. Additionally, the combination of sirolimus plus MMF and a calcineurin inhibitor has been shown to significantly decrease GVHD in the reduced-intensity setting compared with [the effect observed with] MMF and a calcineurin inhibitor alone.2
CAR [chimeric antigen receptor] T-cell–antibody therapy plus antithymocyte globulin [ATG] and alemtuzumab [Lemtrada] are more frequently used in Europe [than in the United States]. There have been mixed results, and there is some concern of increased relapse with [anti-thymocyte globulin (ATG) therapy]. Ex vivo T-cell depletion and CD34-positive cell selection [are] also uncommon in the United States.
Posttransplant cyclophosphamide [Cytoxan] [is becoming more common], and it was originally [used in the setting of] haploidentical transplants. Now it is increasingly used in the unrelated donor setting, and I’m sure it will be translated to the sibling setting, too. [This regimen] has been shown to decrease effector T cells.3 Moreover, chronic GVHD is [reduced by this regimen], but aGVHD is not changed.
A recent study retrospectively compared many patients [with haploidentical donors] to a smaller number of patients [who had] unrelated donors and who received posttransplant cyclophosphamide. The data showed that the patients with unrelated donors and posttransplant cyclophosphamide had better overall survival [OS] and decreased relapse compared with the patients with haploidentical donors.4
For a long time, [most trials that compared GVHD and OS between patients with haploidentical] vs unrelated or sibling donors have shown that posttransplant [cyclophosphamide in the setting of haploidentical] transplants is associated with reduced chronic GVHD, but the other outcomes were the same. Is this result attributable to the fact that the transplant is haploidentical, or is it attributable to the posttransplant cyclophosphamide? I think that question will be answered within the next [few] years.
What risk factors for GVHD do you notice in the case described?
There are multiple risk factors. The fact that the donor is multiparous puts the recipient at higher risk for GVHD. The patient’s high intensity, myeloablative conditioning regimen increases the risk for GVHD, as do the donor’s CMV seropositivity and the fact that the patient and donor are not sex matched. The risk of GVHD also increases with donor age.
Risk for GVHD is also increased by major human leukocyte antigen [HLA] disparity. We look at class I [HLA-A, -B, and -E] and class II [HLA-DR and -DQ] antigens, with a 10 out of 10 score constituting a match. There are data coming out that show that the class II antigen HLA-DP also matters in certain cases5; a match that includes this antigen [a 12 out of 12 (score)] is better than a 10 out of 10 [score]. [This patient’s donor was HLA-matched, but] minor HLA mismatches can increase the risk of GVHD [in patients like this one whose donor is unrelated].
Stem cell source and graft composition are other considerations, but this patient received peripheral blood, which confers a higher risk of GVHD than does bone marrow. Peripheral blood has a higher CD34-positive cell count, therefore a higher T-cell dose; both factors increase GVHD risk. At our center, we don’t often cap CD34-positive cell count or T-cell dose, except in the haploidentical setting.
I would not include ABO blood type as a risk factor. There are mixed data regarding whether major and minor ABO mismatches lead to increased GVHD.6
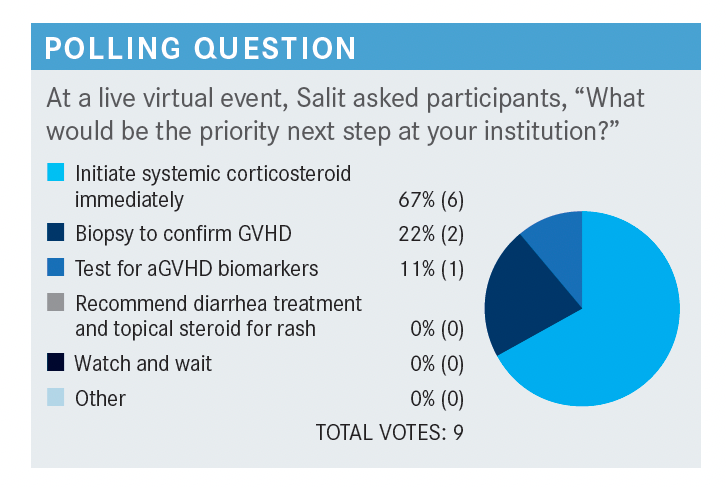
What standardized guidelines exist for organ staging and grading in the context of aGVHD?
[According to Mount Sinai Acute GVHD International Consortium], the skin, the liver, and gastrointestinal [GI] tract are the 3 organs included in aGVHD staging. The skin is [described in terms of] the percentage of body surface area [BSA] affected. Stage 0 is no rash, stage 1 is a rash covering less than 25% of the BSA, stage 2 is a rash covering 25% to 50% of the BSA, stage 3 is a rash covering greater than 50% of the BSA, and stage 4 is generalized erythroderma.7
According to the liver status [bilirubin level], staging starts at stage 0 [less than 2 mg/dL] and progresses through stage 1 [2-3 mg/dL], stage 2 [3.1-6 mg/dL], and stage 3 [6.1-15 mg/dL] to a final stage of 4 [greater than 15 mg/dL]. The lower GI staging system [counts] the number of episodes per day of liquid stool output. Stage 0 is fewer than 3 episodes [of stool output], stage 1 is 3 to 4 episodes, stage 2 is 5 to 7 episodes, and stage 3 is greater than 7 episodes. If you have an inpatient, then you can use these exact quantities. If you have an outpatient, you can use these values as rough markers. Regarding the upper GI staging system, in stage 0, nausea, vomiting, or anorexia are absent or intermittent, but in stage 1, they are persistent.
The other thing I often look at [to judge] severity [of GI involvement] is the electrolytes. For example, if the patient says they are having 5 episodes of stool a day, but their potassium and magnesium are normal and they’re not becoming acidemic, then you [might consider that] these stools are only of small volume. If the patient starts to have electrolyte abnormalities or starts to become acidemic, then you [should consider that] maybe they’re having more diarrhea than they’re [telling you].
When we grade according to most severe target organ involvement, grade I reflects the presence only of stage 1 to 2 skin involvement. Any GI or liver involvement is automatically [at least grade] II, the point at which you would consider treating symptoms topically. Grade IIA indicates upper GI involvement, and grade IIB indicates lower GI involvement.8 Once the patient gets to grade III, they almost always [require] systemic therapy.7

What stage and grade would you give this patient?
With 60% skin involvement, he would have a skin stage of 3, and with 4 episodes of diarrhea per day, he would have a lower GI stage of 1. [He would have an overall clinical grade of IIB.]
What are GVHD and aGVHD biomarkers, and how are they used?
Biomarkers of GVHD are markers of inflammation found in the blood that will tell you the patient is at a higher risk for developing GVHD. These biomarkers include elafin, IL-2 receptor-α, IL-8, tumor necrosis factor receptor-1, hepatocyte growth factor, and regenerating islet-derived 3-α [NCT00224874].9 The use of biomarkers [to predict patients’ risk of developing GVHD could guide physicians as they choose] a starting steroid dosage, eg, 2 mg/kg vs 1 mg/kg.
What data guide your decisions about steroid therapy in GVHD?
Although the concept of GVHD and aGVHD risk stratification is not generally used in practice, high-risk GVHD vs standard-risk GVHD has been shown to be associated with a lower rate of complete response to steroids [27% vs 48%, respectively; P < .001] and higher treatment-related mortality [incidence at 6 months after steroid therapy onset, 44% vs 22%, respectively; P < .001].10 If a patient has a higher grade of GVHD, they are more likely to be steroid refractory.
The steroid response of GVHD is classified as “steroid refractory or resistant” if GVHD progresses within the first 3 to 5 days of prednisone therapy onset [≥ 2 mg/kg per day], fails to improve within 5 to 7 days of treatment initiation at 1 mg/kg or shows an incomplete response after more than 28 days [of immunosuppressive treatment including steroids]. “Steroid dependence” [means that either] the prednisone cannot be tapered below 2 mg/kg daily or the GVHD recurs during the steroid taper.11
You can’t really [know] who is going to respond to steroids without [trying]. Our initial treatment for any patient with GVHD is steroids. There are no data to suggest that we [should] add something other than steroids as the first line or that we [should] add double therapy for the first line. It’s going to be different for every individual.
Also, regarding steroid therapy, the question has been raised: If patients receive higher doses sooner, will that result in a lower [total] exposure to steroids? In the study we did at our institution, we found that when patients with skin GVHD were randomly assigned [to receive] 1 mg/kg vs 0.5 mg/kg, patients [who received the lower dose] had a longer and higher overall exposure to steroids.12 [In cases of skin GVHD], we tend to undertreat patients, and it may help to give them at least 1 mg/kg, but for GI GVHD, we usually give 1 mg/kg. It may not help to give 2 mg/kg unless the GVHD is severe.

Other than steroids, what therapy options exist for aGVHD, according to the National Comprehensive Cancer Network (NCCN)?
Ruxolitinib [Jakafi] is the only approved therapy, and it is supported by category 1 evidence. Some other therapies, such as MMF and sirolimus, are [relatively] benign. Other treatments, like ATG, are more toxic, whereas extracorporeal photopheresis [ECP] doesn’t have a lot of data [to support it]. However, we do use a lot of ECP, [primarily for] steroid-dependent GVHD of the skin.13
What data support the use of ruxolitinib for aGVHD?
In the REACH1 study [NCT02953678], patients with steroid refractory grade 2 to 4 aGVHD received ruxolitinib, 5 mg twice a day. Later, patients could increase to 10 mg twice a day.14,15
The overall response rate [ORR] at day 28 was about 55%. The best ORR at any time during treatment was 73%. Time to response was about 7 days [range, 6-49]. The median duration of response was almost a year. Death from causes other than malignancy relapse was found in about 50% of patients. The median OS was about 5 months, whereas median OS for steroid refractory GVHD was 1 month, [but median OS for day 28 responders was not reached].16,17 The overall response rate [ORR] at day 28 was about 55%. The best ORR at any time during treatment was 73%. Time to response was about 7 days [range, 6-49]. The median duration of response was almost a year. Death from causes other than malignancy relapse was found in about 50% of patients. The median OS was about 5 months, whereas median OS for steroid-refractory GVHD was 1 month, [but median OS for day 28 responders was not reached].15
The ORR at day 28 was 62% in the ruxolitinib group vs 39% in the control group [odds ratio (OR), 2.64; 95% CI, 1.65-4.22; P < .001]; the durable ORR at day 56 was 40% in the ruxolitinib group vs 22% in the control group [OR, 2.38; 95% CI, 1.43-3.94; P < .001].18 These results led to the FDA approval of ruxolitinib for second-line therapy for steroid-refractory aGVHD.19
[Separate analyses were conducted of] GI and skin GVHD. In the ruxolitinib group, aGVHD staging of the lower GI was stage 3 and 4 for most patients at baseline. This was reduced in most patients to stage 0, 1, and 2 by day 28. In contrast, most patients treated with BAT still presented with stage 2 to 4 GVHD by day 28. Likewise for the skin, the GVHD stage was more likely to decrease following treatment with ruxolitinib than with BAT.19
Median failure-free survival was 5 months in the ruxolitinib group vs 1 month in the BAT group [HR, 0.46; 95% CI, 0.35-0.60]; 5 months was a big achievement compared with our previous standard. After 1 year, 40% of the patients in the experimental group were still alive. Regarding AEs associated with ruxolitinib, the most difficult [AE to manage] is thrombocytopenia [in REACH2, affecting 33% of the ruxolitinib group vs 18% of the BAT group]. Infections with ruxolitinib [in the context of GVHD] probably are equivalent to [those observed with] any other immune suppression drug [for cytomegalovirus, 26% in the ruxolitinib group, 21% in the BAT group].19
REFERENCES:
1. Yoshida S, Ohno Y, Nagafuji K, et al. Comparison of calcineurin inhibitors in combination with conventional methotrexate, reduced methotrexate, or mycophenolate mofetil for prophylaxis of graft-versus-host disease after umbilical cord blood transplantation. Ann Hematol. 2019;98(11):2579-2591. doi:10.1007/s00277-019-03801-z
2. Bejanyan N, Rogosheske J, DeFor TE, et al. Sirolimus and mycophenolate mofetil as calcineurin inhibitor-free graft-versus-host disease prophylaxis for reduced-intensity conditioning umbilical cord blood transplantation. Biol Blood Marrow Transplant. 2016;22(11):2025-2030. doi:10.1016/j. bbmt.2016.08.005
3. Włodarczyk M, Ograczyk E, Kowalewicz-Kulbat M, Druszczyńska M, Rudnicka W, Fol M. Effect of cyclophosphamide treatment on central and effector memory T cells in mice. Int J Toxicol. 2018;37(5):373-382.
4. Shaw BE. Related haploidentical donors are a better choice than matched unrelated donors: counterpoint. Blood Adv. 2017;1(6):401-406. doi:10.1182/bloodadvances.2016002188
5. Zachary AA, Leffell MS. HLA mismatching strategies for solid organ transplantation - a balancing act. Front Immunol. 2016;7:575. doi:10.3389/ fimmu.2016.00575
6. Brierley CK, Littlewood TJ, Peniket AJ, et al. Impact of ABO blood group mismatch in alemtuzumab-based reduced-intensity conditioned haematopoietic SCT. Bone Marrow Transplant. 2015;50(7):931-938. doi:10.1038/bmt.2015.51
7. Harris AC, Young R, Devine S, et al. International, multicenter standardization of acute graft-vs-host disease clinical data collection: a report from the Mount Sinai Acute GVHD International Consortium. Biol Blood Marrow Transplant. 2016;22(1):4-10. doi:10.1016/j.bbmt.2015.09.001
8. Lee SJ. Classification systems for chronic graft-versus-host disease. Blood. 2017;129(1):30-37. doi:10.1182/blood-2016-07-686642
9. Levine JE, Logan BR, Wu J, et al. Acute graft-vs-host disease biomarkers measured during therapy can predict treatment outcomes: a Blood and Marrow Transplant Clinical Trials Network study. Blood. 2012;119(16):3854-3860. doi:10.1182/blood-2012-01-403063
10. MacMillan ML, Robin M, Harris AC, et al. A refined risk score for acute graft-vs-host disease that predicts response to initial therapy, survival, and transplant-related mortality. Biol Blood Marrow Transplant. 2015;21(4):761-767. doi:10.1016/j.bbmt.2015.01.001
11. Schoemans HM, Lee SJ, Ferrara JL, et al; European Society for Blood and Marrow Transplantation [EBMT] Transplant Complications Working Party; EBMT-National Institutes of Health [NIH]-Center for International Blood and Marrow Transplant Research [CIBMTR] GVHD Task Force. EBMT-NIH-CIBMTR Task Force position statement on standardized terminology & guidance for graft-vs-host disease assessment. Bone Marrow Transplant. 2018;53(11):1401-1415. doi:10.1038/s41409-018-0204-7
12. Mielcarek M, Furlong T, Storer BE, et al. Effectiveness and safety of lower dose prednisone for initial treatment of acute graft-versus-host disease: a randomized controlled trial. Haematologica. 2015;100(6):842-848. doi:10.3324/haematol.2014.118471
13. NCCN. Clinical Practice Guidelines in Oncology. Hematopoietic cell transplantation, version 5.2021. Accessed October 13, 2021. https://www.nccn.org/professionals/physician_gls/pdf/hct.pdf
14. Chao N. Finally, a successful randomized trial for GVHD. N Engl J Med. 2020;382(19):1853-1854. doi:10.1056/NEJMe2003331
15. Jagasia M, Zeiser R, Arbushites M, Delaite P, Gadbaw B, von Bubnoff N. Ruxolitinib for the treatment of patients with steroid-refractory GVHD: an introduction to the REACH trials. Immunotherapy. 2018;10(5):391-402. doi:10.2217/ imt-2017-0156
16. Jagasia M, Perales MA, Schroeder MA, et al. Ruxolitinib for the treatment of steroid-refractory acute GVHD (REACH1): a multicenter, open-label phase 2 trial. Blood. 2020;135(20):1739-1749. doi:10.1182/blood.2020004823
17. Jagasia M, Ali H, Schroeder MA, et al. Ruxolitinib in combination with corticosteroids for the treatment of steroid-refractory acute graft-vs-host disease: results from the phase 2 REACH1 trial. Biol Blood Marrow Transplant. 2019;25(suppl 3):S52. doi:10.1016/j.bbmt.2018.12.130
18. Zeiser R, von Bubnoff N, Butler J, et al; REACH2 Trial Group. Ruxolitinib for glucocorticoid-refractory acute graft-vs-host disease. N Engl J Med. 2020;382(19):1800-1810. doi:10.1056/NEJMoa1917635
19. Przepiorka D, Luo L, Subramaniam S, et al. FDA approval summary: ruxolitinib for treatment of steroid-refractory acute graft-versus-host disease. Oncologist. 2020;25(2):e328-e334. doi:10.1634/theoncologist.2019-0627
