Chao Discusses Different Staging Systems for Acute GVHD
During a Targeted Oncology Case-Based Roundtable event, Nelson Jen An Chao, MD, a discussed the case of a young patient with acute graft-versus-host disease.
Nelson Jen An Chao, MD

During a Targeted Oncology Case-Based Roundtable event, Nelson Jen An Chao, MD, a research professor of Global Health Professor of Medicine, Immunology, and Pathology, the Donald D. and Elizabeth G. Cooke Cancer Distinguished Research Professor Member atDuke Cancer Institute Chief, Division of Cell Therapy in the Department of Medicine Duke University School of Medicine, discussed the case of a young patient with acute graft-versus-host disease.

Targeted OncologyTM: What are the patient’s risk for developing acute GVHD?
CHAO: I’m going to guess some of the issues around this. … CMV seropositivity is a problem because that’s associated with increased GVHD. Age is a problem, as older patients don’t do as well as kids. And women who’ve had multiple children have a higher risk of acute GVHD; as a donor [they have a higher risk] of causing more GVHD, mainly because they’ve been exposed to more HLAs [human leukocyte antigen].
What are some of the other risks for acute GVHD?
It turns out that there have been a lot of different risk factors, [including] donor and recipient factors. Obviously the most common one is HLA disparity, and it has been known since the late 1940s that HLA differences, the so-called major histocompatibility differences, caused a lot of GVHD. So HLA mismatched donors cause a lot more GVHD than matched donors. Now, we also know that there are minor HLA disparities, so these minor antigens are not structural proteins but…peptides that are presented by the MHC [major histocompatibility complex] class I and class II molecules. That’s why unrelated donors have a higher risk of acute GVHD than related donors.
An unrelated donor that’s HLA matched…should be the same as a related donor but they’re not, because the minor antigens, the antigens presented by those MHC molecules, are not restricted by the HLA, by the chromosome where the HLA is. These are unrelated minor antigens, [as in] how one person “chops up” transferrin, for example.
Sex match is important, so a mismatch is a higher risk than matched. Donor parity is important, so women that are multiparous, or [who] have had multiple children as a donor, have a higher risk of causing GVHD. Donor age is important; older donors are much more likely to cause GVHD than younger donors. ABO type is important, as some ABO mismatch causes more GVHD than ABO match. This tends not to be a common issue that we focus on much but it certainly has been shown to be a problem. CMV status is probably one of the more potent ones, so donors that are CMV positive, especially [regarding] a CMV-negative recipient, can be problematic because they sometimes don’t respond with a CMV response. There are multiple cytokine gene polymorphisms that have been described that have been associated with acute GVHD.
We do have very good data from prospective randomized phase 3 trials that peripheral blood causes more GVHD than bone marrow. Not so much from randomized studies but case studies and meta-analysis, [the data show that] the cells that cause the least amount of GVHD [are those of the] umbilical cord.
Graft composition makes a difference, or more CD34s cause more GVHD, and this may be related to the other cells that come in with the CD34 count. So a higher T-cell dose certainly causes more GVHD. That’s not surprising, of course, because the T cells are the cells causing GVHD. And lastly, the transplantation factors can be an issue, obviously, because MAC causes a lot more tissue damage. [According to] James Ferrara, [MD]’s circle of conditioning, the more ablative or the more intensive the conditioning, the more likely you’re going to end up with cytokine release and tissue damage.1
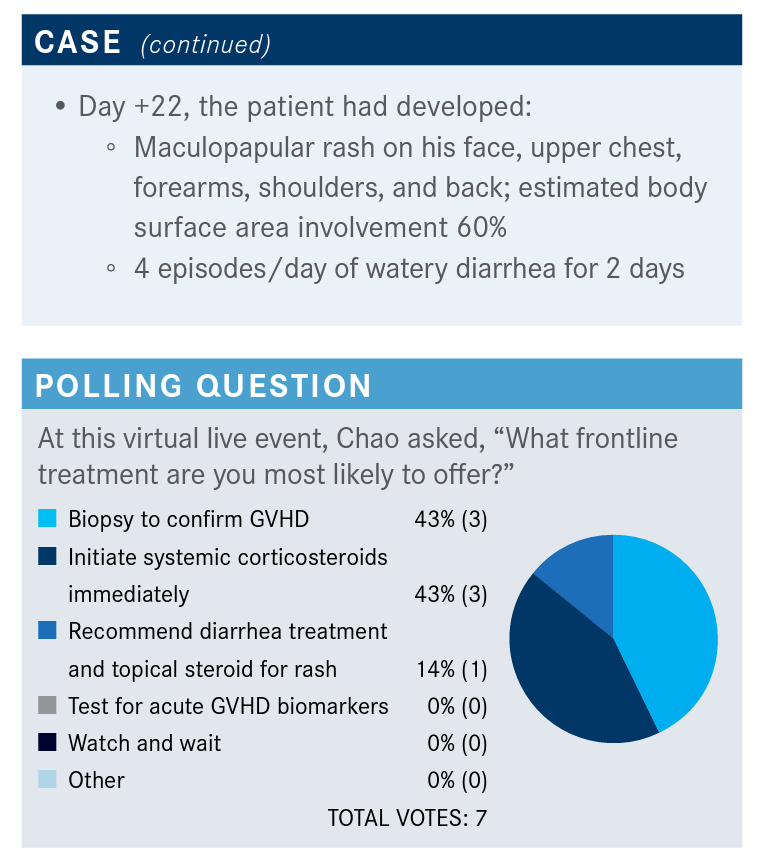
What do you think of the responses to the poll?
So, about half…would confirm GVHD and about half would initiate steroids, which is probably what most of us would do. It is interesting because I don’t think there’s anything wrong with starting steroids; I think the concern of starting steroids without a biopsy is that you really don’t have a good sense of how long to continue the steroids for. The flip side of that is—there have been several studies, including one we did many, many years ago when I was at Stanford—that if you send a biopsy from a transplant unit, the likelihood that the pathologist is going to call GVHD is extremely high.
At day 22 post transplant, the honest to goodness truth is most pathologists cannot tell the difference between what is…truly GVHD vs toxicity of the regimen. Because when you send these [biopsies] blind—if you take the same set of slides that went from the pathologist and you blind the pathologist without knowing where that slice [came] from or the post transplant day—most pathologists really can’t tell the difference.
What are the institutional preferences for staging acute GVHD?
I think most places have latched on to this MAGIC [Mount Sinai Acute GVHD International Consortium].2 It’s really not very different from what we’ve been using for many years, from the Glucksberg [scale] to the IBMTR [International Bone Marrow Transplant Registry]. The major difference is really around the GI [gastrointestinal] tract, which is adding the nausea, vomiting, or anorexia. In the past, the upper GI was not incorporated into any GVHD grading. I think it became clear, probably about 15 years ago, that the upper GI tract does get affected by GVHD, and the manifestation of that is really nausea and vomiting. There is a clear pathologic, actually a visible, change on endoscopy in a pathologic finding in the upper GI tract. And the lower GI has changed, with not just volume but multiple episodes of diarrhea.
So overall, grade 0 is pretty straightforward, which means there’s none. Grade 4 is pretty obvious too, which is basically grade 4 of any of the major organs, and grades 2 and 3 are somewhere in the middle. If you spend some time looking at the grading, you understand why GVHD is a problem because it’s not very hard at all to recognize what grade 0 and grade 1 are, or what grade 4 is. It is much more difficult to recognize what grade 2 is, because if you get a rash or get a little bit of bilirubin elevation or a little bit of diarrhea, as many who treat these patients know, there are probably at least a dozen reasons for many of these manifestations. Whether it’s a drug, …a virus, or…part of the prep regimen, or whether the patient had a burrito the day before, there are [endless] potential reasons why some of these symptoms might occur. And because GVHD’s a clinical grading system, and it’s a clinical call, you can understand why some of these could be misconstrued as to whether this is truly GVHD, a drug toxicity, infection, or just something the patient ate the day before.
What is the prognosis for these patients?
One of the things that’s been very interesting recently is this paper by Maggie MacMillan, [MD, and colleagues,] at Minnesota, who actually took over a thousand patients they’ve seen and risk stratified the patients who were categorized as acute GVHD.³ That dataset allowed them to [break the numbers down] into 2 scores: a standardrisk score and a high-risk score. [They] took the amount of damage or the amount of symptoms per organ and… split out a risk based on the level of damage per organ in that population and added what the impact was of adding a second or third organ.
Looking at standard risk, …you would not be surprised, for example, [that patients with] skin rash only up to grade 3, or GI only up to grade 1 and 2, really do reasonably well. I would say that most of us probably would not expect that stages I to IV liver complications—that would be under 2 organs—would be standard risk. I think most of us would have thought that if you had liver problems, you would not do well.
Anybody with higher risk of any of the organs, potentially, except for the skin, really does quite poorly, because more than 1 organ involvement with grade 3 disease tends to portend a poor prognosis.
And what [MacMillan et al were] able to do was to clearly show that higher-risk GVHD is associated with a lower complete response [CR] rate to steroids and also a higher treatment-related mortality. The percent response for those who have standard risk [69%] is much higher than those who have high risk [43%], both as CRs and PRs [partial responses]. And the probability of treatment-related mortality is double if you have high risk vs standard risk [44% vs 22%]. So this allows you, at the time you’re [giving] these patients [a diagnosis], to risk stratify them to who’s going to do okay with steroids and who’s not.
[Also] quite interesting was to look at biomarkers [in] John Levine, [MD]’s data.4 We have been trying for many, many years to come up with biomarkers because, as I said earlier, the major problem with GVHD is that it’s clinical. And because it’s clinical, it tends to be in the eye of the beholder. So what may be a 49-skin involvement vs a 51-skin involvement from observer to observer might vary quite a bit. And whether you blow off a bilirubin of 2 to Gilbert [syndrome] or to GVHD might be very different from clinician to clinician.
These are data [from studies that have] looked at multiple biomarkers like elafin, IL [interleukin]-2 receptor-α, TNFR1 [tumor necrosis factor 1], hepatocyte growth factor, IL-8, and REG3-α—and shown that there’s a difference in survival in some of these biomarkers, like REG3-α, IL-8, TNFR1, and elafin.

What is the expected response to steroids? We tend to think of this as an algorithm or a framework [for being] steroid refractory or [for] resistance. And it does make a difference, primarily because of studies, because you have to have a common definition. [Patients move on if there is] progression of acute GVHD within 3 to 5 days of therapy onset with 2 mg/kg of prednisone, if they don’t improve in 5 to 7 days of the treatment or if they have an incomplete response after more than 28 days of steroids. So, either of those could be thought of as refractory or resistant.
The second bucket of people are those who you can’t taper. So they get 2 mg/kg and they get better; and as you try to taper them, they flare again. I think that’s a difficult group as well. Or you have a patient [who received] 1 mg/kg or 2 mg/kg and got better, and you taper them down from 2 to 1½ to 1 and they flare again. That’s thought to be steroid dependence. And obviously steroid intolerance is pretty standard. We know a lot of these patients where they just can’t stand it. Patients go crazy; they have psychotic issues or their sugars go to 800 [mg/dL] and that becomes problematic.
What systemic treatment options are available for patients with steroid-refractory GVHD?
If you look at the NCCN [National Comprehensive Cancer Network guidelines], the primary suggested therapy for second line is clinical trials.⁵ That’s not really surprising. Outside of steroids we really didn’t have clear indications until the past 2 years. Last year, ruxolitinib [Jakafi] was approved in acute [GVHD], and about a year and a half ago, ibrutinib [Imbruvica] was approved for chronic. But outside of that, over the past 30 to 40 years, it’s really been the kitchen sink. [There are] multiple different agents, many of them with very different mechanisms of action, that have been tried for both acute and chronic [GVHD].
What data support the use of ruxolitinib in acute GVHD?
The phase 2 trial [REACH-1, NCT02953678] was the first trial, and the concept for using ruxolitinib was to try to block STAT signaling through the dendritic cells to induce a drop in cytokines, which are inflammatory in these TH17 cells. TH17 cells tend to be a cell in the gut, which induces inflammation, so the idea is to try to drop the inflammatory response and at the same time inhibit the TH1 response, which is the pro-inflammatory response.6
This first study was with 71 patients with steroid-refractory acute GVHD, who [received] ruxolitinib 5 mg twice a day with steroids plus or minus calcineurin inhibitor.⁷ This [included] any patients with grade 2 to 4 acute GVHD who progressed after 3 days, or had no response after 7 days, or could not be tapered. The primary end point was overall response at day 28. They started therapy and 4 weeks later, were they better or were they not?
There was a whole bunch of exploratory end points such as overall response, duration of response, incidence of GVHD, safety, and so forth. They started therapy and 4 weeks later, were they better or were they not? And, there was a whole bunch of exploratory end points such as overall response, duration of response, incidence of GVHD, safety, and so forth. The overall response rate was about 55%, with about half of those being CRs. The best overall response rate was 73%. Time to response was 7 days, so it was relatively quick. Duration of response was about a year. As you can imagine, with acute GVHD, a significant number of deaths [occurred] (49.3%). The nonrelapse mortality rate was 44.4%, and the median overall survival at that point, for those that responded at day 28, was not reached.
This was quite encouraging, and led…to the phase 3 REACH2 trial [NCT02913261].8 Essentially, it was the same type of trial, using now ruxolitinib at 10 mg twice a day for those who were steroid refractory. [Patients] were randomized to best alternative therapy—they left this open to the investigator—and the selection was quite broad: you could use ATG [antithymocyte globulin], …ECP [extracorporeal photopheresis], …mesenchymal stromal cells, low-dose methotrexate, MMF [mycophenolate mofetil], everolimus [Afinitor], sirolimus [Rapamune], etanercept [Enbrel], or infliximab [Remicade]. You were not to use any other [therapy] but you had to use one of those. Almost 300 patients [were] randomized. And to their credit [the investigators] allowed crossover for those [who progressed on] best alternative therapy. Obviously, the downside of crossover is you really don’t have a clear picture of how good the drug really is, because [in] those who cross over, if they respond, you really can’t compare it as well.
The primary end point, overall response rate, at day 28 was markedly better for ruxolitinib: 62% over 39%, and the odds ratio was 2.64. Most of this was durable: at day 56, about 40% of those patients were still responding compared with [22% in the control arm], with an odds ratio of 2.38.
If you look at the stage, it was relatively consistent. So if you took everybody, the odds ratio of CR/PR was markedly higher for ruxolitinib. And for each grade, the odds ratio is in favor of being treated with ruxolitinib, with [the] ratio ranging from between 2.15 to 3.76.
One of the more important things with this study was that the duration of response was pretty impressive. … With many of these types of studies you get an initial response that you lose over time. In this case, it really was maintained. In the Kaplan-Meier curve, the percentage of patients who stayed controlled on ruxolitinib really did seem to [respond] for over a year.
One of the ways to look at this is to look at what the shift is from baseline to the end of the study [in terms of organ staging]. From ruxolitinib before and after, you can see that there’s very little [stage IV in the skin]. If you look at the GI tract, ruxolitinib before and after, [there is] very little [stage IV]. And if you then look in the lower GI tract, there’s less [stage IV] in the ruxolitinib compared to best alternative therapy. So there is a shift in the organ staging to lower grade or lower stage.
The failure-free survival obviously was much better with ruxolitinib. The hazard ratio was 0.46, meaning that if you got control of the disease, most of these patients did significantly better than [those in the] control. So patients who responded did not fail at the same level as those in the control.
We’ve all used ruxolitinib in other settings, so that was one of the benefits of this drug. We know it causes thrombocytopenia. There are some infectious concerns, obviously, as you induce neutropenia as well with ruxolitinib, but overall it was remarkably well tolerated. If you look at the AEs [adverse events] compared to the control group with the best alternative therapy, there really was not a huge difference outside of thrombocytopenia.
References:
1. Hill GR, Ferrara JLM. The primacy of the gastrointestinal tract as a target organ of acute graft-versus-host disease: rationale for the use of cytokine shields in allogeneic bone marrow transplantation. Blood. 2000;95(9):2754 2759. doi:10.1182/blood. V95.9.2754.009k25_2754_2759
2. Harris AC, Young R, Devine S, et al. International, Multicenter Standardization of Acute GVHD Clinical Data Collection: A Report from the Mount Sinai Acute GVHD International Consortium. Biol Blood Marrow Transplant. 2016;22(1):4-10. doi:10.1016/j. bbmt.2015.09.001
3. MacMillan ML, Robin M, Harris AC, et al. A refined risk score for acute graft-versus-host disease that predicts response to initial therapy, survival, and transplant-related mortality. Biol Blood Marrow Transplant. 2015;21(4):761-767. doi:10.1016/j.bbmt.2015.01.001
4. Levine JE, Logan BR, Wu J, et al. Acute graft-versus-host disease biomarkers measured during therapy can predict treatment outcomes: a Blood and Marrow Transplant Clinical Trials Network study. Blood. 2012;119(16):3854-3860. doi:10.1182/ blood-2012-01-403063
5. NCCN Clinical Practice Guidelines in Oncology. Hematopoietic Cell Transplantation (HCT): Pre-Transplant Recipient Evaluation and Management of Graft-Versus-Host Disease, version 2.2021. April 21, 2021. Accessed July 8, 2021. https://www.nccn.org/ professionals/physician_gls/pdf/hct.pdf
6. Jagasia M, Zeiser R, Arbushites M, Delaite P, Gadbaw B, von Bubnoff N. Ruxolitinib for the treatment of patients with steroid-refractory GVHD: an introduction to the REACH trials. Immunotherapy. 2018;10(5):391-402. doi:10.2217/imt-2017-0156
7. Jagasia M, Perales MA, Schroeder MA, et al. Ruxolitinib for the treatment of steroidrefractory acute GVHD (REACH1): a multicenter, open-label phase 2 trial. Blood. 2020;135(20):1739-1749. doi:10.1182/blood.2020004823
8. Zeiser R, von Bubnoff N, Butler J, et al; REACH2 Trial Group. Ruxolitinib for GlucocorticoidRefractory Acute Graft-versus-Host Disease. N Engl J Med. 2020;382 (19):1800-1810. doi:10.1056/NEJMoa1917635
