Combination of Tafasitamab/Lenalidomide Demonstrates Positive Efficacy in DLBCL
The laboratory results, lymph node biopsy, imaging studies and other information of a 75-year-old patient with diffuse large B-cell lymphoma were brought into consideration during a Targeted Oncology Case-Based Roundtable discussion around treatment with tafasitamab and lenalidomide (Revlimid).

The laboratory results, lymph node biopsy, imaging studies and other information of a 75-year-old patient with diffuse large B-cell lymphoma were brought into consideration during a Targeted Oncology Case-Based Roundtable discussion around treatment with tafasitamab and lenalidomide (Revlimid).
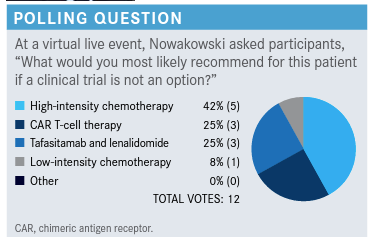
Targeted OncologyTM: Please discuss the poll results and what the recommendations are from National Comprehensive Cancer Network (NCCN) guidelines.
NOWAKOWSKI: About a quarter of [participants] would recommend CAR [chimeric antigen receptor] T-cell therapy; [they] are telling us that it even can be done in older patients, so that’s an option at least to consider depending on what the factors were where he was not a candidate for transplant in the first place. Low-intensity chemotherapy was not very popular, only about 8%. High-intensity chemotherapy was 42% and the combination of tafasitamab [Monjuvi] plus lenalidomide [Revlimid] was 25%. So, about a quarter of you were considering this combination for this patient, who is not transplant eligible.
The current NCCN guidelines for second-line therapy for DLBCL [say the] preferred chemotherapy regimens, which have been studied extensively, include gemcitabine [Gemzar] and oxaliplatin [Eloxatin] with or without rituximab [Rituxan] and polatuzumab vedotin [Polivy], bendamustine [Treanda], and rituximab [pola-BR].1 There are a number of other [recommended regimens] including GDP [gemcitabine, dexamethasone, carboplatin], which is a popular choice.1 There are other gemcitabine chemotherapy combination therapies used. I don’t typically use single-agent rituximab, but it is listed as an option by the NCCN.
Recently, tafasitamab plus lenalidomide was added based on the results from the L-MIND study [NCT02399085].1,2 This combination has been approved to treat patients with relapsed/refractory DLBCL who are not eligible for stem cell transplantation.1
Interestingly, the approval for pola-BR is for 2 or more lines of therapy; so, relapse after second-line therapy, but some people use it in this setting as well.

What are the efficacy and safety of the combination of tafasitamab and lenalidomide?
As I mentioned, the combination tafasitamab and lenalidomide was approved by the FDA in July 2020.3 Its accelerated approval was based on the activity of this combination for relapsed/refractory DLBCL.4 The mechanism of action of this combination is very interesting. We know that lenalidomide synergizes with different antibodies. The observation of high synergy with rituximab in 2 combinations used for low-grade lymphoma led to the development of this study, where the CD19 antibody [tafasitamab] was combined with lenalidomide.2
Tafasitamab is a very interesting antibody. It targets CD19 and is engineered to have a fragment crystallizable- enhanced induction of antibody-dependent cellular cytotoxicity, antibody-dependent cellular phagocytosis, and direct cell death via B-cell receptor signaling.5 As a single agent, tafasitamab showed quite a reasonable activity of about 20% to 30%, which is similar to many other single agents. What makes tafasitamab outstanding is the combination with lenalidomide, and we believe that this is due to the mode of action of lenalidomide, where lenalidomide activates T and natural killer cells, but also causes direct cell death and affects the immune microenvironment.
Lenalidomide is approved for the treatment of mantle cell lymphoma, follicular lymphoma, and marginal zone lymphoma, typically as part of a 2-drug combination. The L-MIND study combined lenalidomide [25 mg daily], day 1 through 21 of 4-week cycles; this is the standard dose used to treat lymphoma, instead of 20 mg, which is the dose for [lenalidomide plus rituximab].6 Tafasitamab was given initially with a loading schedule, on days 1, 8, 15, 22, for cycles 1 through 3.5 Then, for cycles 4 to 12, it was given on days 1 and 15. After 12 cycles of therapy, patients who had stable disease continued with tafasitamab as a single-agent therapy. The reasoning for this was to keep it as an option for patients who were benefitting from this therapy as there are very limited treatment options.
The inclusion criteria were relapsed/refractory large cell lymphoma treated with 1 to 3 prior regimens containing rituximab, which were not eligible for local stem-cell transplantation. The primary end point was the overall response rate [ORR], with the secondary end points being progression-free survival [PFS], duration of response [DOR], overall survival [OS], safety profile, and exploratory and biomarker-based analyses.
The following were the characteristics of the study participants [n = 81].2 Most patients were older [median age, 72 years (range, 62-76)] as is expected from a cohort not eligible for transplantation. Participants had a fairly high International Prognostic Index risk score and advanced Ann Arbor stage. A good proportion had elevated LDH, and had received between 1 and 4 prior lines of therapies. A fair number of patients [n = 19] had primary refractory disease. There were also a number of patients [44] who were refractory to the last prior line of therapy. A small proportion of patients [11] had a prior stem-cell transplant. Additionally, a cell-of-origin assessment was done for some of the patients, but it was not a major contributor to the activity of this combination. This is because this combination probably works mostly on the microenvironment, rather than direct cell killing, which is seen with lenalidomide alone.
The ORR was very exciting for this combination. At 17 months the complete response [CR] rate was 43% and an additional 18% of patients had a partial response [PR].2 This represents a relatively high response rate. These rates were similar [CR, 41.3%; PR, 17.5%] at the 2-year followup. This means that about 60% of patients respond to this combination, which in relapsed/refractory lymphoma is quite outstanding and this is what led to the FDA approval. The median DOR at the initial report was 21 months and it increased to 34 months at the 2-year follow-up. This is an outstanding DOR. PFS was 50% at 12 months and nearly 50% at 18 months, and OS was 74% at 12 months and 64% at 18 months. In comparison, the SCHOLAR-1 study, [a retrospective analysis of 2 large randomized trials and 2 academic databases], found that the median survival for relapsed/refractory large cell lymphoma was about 6 months.7 At the 2-year follow-up, PFS improved to 16.2 months, and the median OS was over 13 months, which is outstanding.4 We don’t know how long this will last there is hope that it goes beyond 5 years and that some patients will not relapse. But, certainly, the PFS plateaus are very encouraging, indicating that the disease control with this combination is quite durable.
The most common [≥20%] hematological adverse effects [AEs] were neutropenia [27% and 21% for grade 3 and 4, respectively] and thrombocytopenia [12% and 5% for grade 3 and 4, respectively].2 Patients can be supported with a growth factor, if needed. This was allowed during the study, and it’s commonly used in practice. Common [≥20%] non-hematological AEs were fatigue, diarrhea, cough, and pyrexia. Overall, the combination was well tolerated, considering the duration of therapy. Importantly, the hematological AEs were not associated with complications like bleeding, and the risk of neutropenic fever was not very high.
Quite a few patients, about 45%, had more than one dose reduction of lenalidomide, and this was typically from 25 mg to 20 mg [77% of patients].
Considering these results, there is a debate on whether to start with a lower dose of lenalidomide or keep it at 25 mg. Since the combination was developed at 25 mg and has been successful, I tend to start my patients at 25 mg and then reduce the dose if they develop cytopenia, meaning that they cannot tolerate it. But I also have a low threshold for initiating growth factor support.
A comparison of the AEs resulting from the combination therapy vs those on monotherapy shows that the antibody by itself, as expected, is well tolerated.5 There is some neutropenia with tafasitamab monotherapy, but it is not very common [<20%]. Most hematological and non-hematological AEs are related to the combination therapy, and that’s typical of what we see with lenalidomide in this setting.
What’s your reaction to safety data for the combination of tafasitamab and lenalidomide?
What is most striking to you? It’s mainly related to the lenalidomide and that’s particularly true about hematologic toxicity. Infusion reactions are quite rare, less than with some of the other CD20 antibodies.
When do you administer the growth factor, in which cycle, and for how long?
Typically, we see toxicity in a pattern that is similar to what happens with lenalidomide [monotherapy], so mid cycle, particularly within the last week of lenalidomide therapy, we initiate filgrastim [Neupogen] to support the patient.
For patients who had poor hematological recovery to begin with, because of therapies that they’d previously received, including CAR-T cell failures, or patients who received filgrastim on several occasions, we use pegfilgrastim [Neulasta].
What is the contribution of tafasitamab in this combination?
The RE-MIND study [NCT04150328] was done to support the regulatory approval of this combination and to determine how much of the activity comes from lenalidomide alone vs the combination.8 The study consisted of a prespecified matching for the following baseline co-variates: age, Ann Arbor stage, refractoriness to last line, number of prior therapies, history of primary refractoriness, prior stem cell transplant, neutropenia, anemia, and elevated LDH. The combination therapy had significantly better ORR, CR, and OS compared to lenalidomide monotherapy. As expected, a third of the patients responded to lenalidomide monotherapy. Based on these results, tafasitamab is a critical contributor to the activity of this combination.
References:
1. NCCN. Clinical Practice Guidelines in Oncology. B-cell lymphomas, version 3.2021. Accessed April 14, 2021. https://bit.ly/3geoS5N
2. Salles G, Duell J, Gonzalez Barca E, et al. Tafasitamab plus lenalidomide in relapsed or refractory diffuse large B-cell lymphoma (L-MIND): a multicentre, prospective, single-arm, phase 2 study. Lancet Oncol. 2020;21(7):978-988. doi:10.1016/S1470-2045(20)30225-4
3. FDA grants accelerated approval to tafasitamab-cxix for diffuse large B-cell lymphoma. FDA. Updated August 3, 2020. Accessed July 6, 2021. https://bit.ly/34Emq2z
4. Salles G, Duell J, Barca-Gonzalez E, et al. Long-term outcomes from the phase 2 study of tafasitamab (MOR208) plus lenalidomide in patients with relapsed or refractory diffuse large B-cell lymphoma. Abstract presented at: 2020 European Hematology Association Congress; May 14, 2020. Accessed July 6, 2021. https://bit.ly/3mVjq9a
5. Salles G, Duell J, Barca-Gonzalez E, et al. Primary analysis results of the singlearm phase II study of MOR208 plus lenalidomide in patients with relapsed or refractory diffuse large B-cell lymphoma (L-MIND). Abstract presented at: 15th International Conference on Malignant Lymphoma; June 18-23, 2019; Lugano, Switzerland. Accessed April 15, 2021. https://bit.ly/3wZgJIt
6. Becnel MR, Nastoupil LJ, Samaniego F, et al. Lenalidomide plus rituximab (R2 ) in previously untreated marginal zone lymphoma: subgroup analysis and longterm follow-up of an open-label phase 2 trial. Br J Haematol. 2019;185(5):874882. doi: 10.1111/bjh.15843
7. Crump M, Neelapu SS, Farooq U, et al. Outcomes in refractory diffuse large B-cell lymphoma: results from the international SCHOLAR-1 study]. Blood. 2017;130(16):1800-1808. doi:10.1182/blood-2017-03-769620
8. Nowakowski GS, Rodgers TD, Marino D, et al. RE-MIND study: A propensity score-based 1:1 matched comparison of tafasitamab + lenalidomide (L-MIND) versus lenalidomide monotherapy (real-world data) in transplant-ineligible patients with relapsed/refractory (R/R) diffuse large B-cell lymphoma (DLBCL). J Clin Oncol. 2020;38(suppl 15):8020. doi: 10.1200/JCO.2020.38.15_suppl.8020

Bispecific Antibodies and ADCs Deliver a Futuristic Horizon Across Lung Cancer Settings
October 23rd 2024Recent advancements in protein engineering, especially antibody-drug conjugates, show promise in lung cancer treatment, with ivonescimab outperforming pembrolizumab in PD-L1-positive advanced non-small cell lung cancer.
Read More