Immune Checkpoint Blockade: A New Strategy for the Treatment of Breast Cancer
Breast cancer has traditionally not been viewed as immunogenic. There is now a growing body of evidence that immune infiltration has a prognostic role in al breast cancer subtypes, and predicts improved clinical outcome in triple-negative and human epidermal growth factor receptor 2-positive tumors.
Abstract
Breast cancer has traditionally not been viewed as immunogenic. There is now a growing body of evidence that immune infiltration has a prognostic role in all breast cancer subtypes, and predicts improved clinical outcome in triple-negative and human epidermal growth factor receptor 2-positive tumors. Immunotherapy is beginning to expand its role as a breast cancer therapeutic. Recent clinical trials of immune checkpoint inhibitors in metastatic breast cancer have shown promising anti-tumor activity. Ongoing studies will help define the role of immune-targeting drugs in the treatment of breast cancer. In this review, we describe the immune environment of breast cancer and summarize the recently reported clinical trial results of immune checkpoint inhibitors in breast cancer.
Introduction
Breast cancer is a molecularly complex and heterogeneous disease that is comprised of biologically distinct subtypes. Important strides have been made during the last decade with the availability of several targeted agents for the treatment of human epidermal growth factor receptor 2 (HER2)-positive breast cancer and for hormone receptor (HR)-positive breast cancer. A portion of patients benefit from such approaches, but resistance frequently develops, and novel treatment options are needed. An increased understanding of the breast tumor immune environment and the nature and role of tumor-infiltrating lymphocytes (TILs) has now led to several clinical investigations of immunotherapeutic approaches in breast cancer.
Infiltration of immune cells, particularly Type I immune cells that are needed to eliminate cancer, has predicted improved prognosis in many different tumor types including colon, ovarian, and lung cancer.1-3The tumor immune environment was historically not thought to be as important in breast cancer because there was less immune infiltrate than in other tumor types such as melanoma.4However, multiple lines of evidence have demonstrated that both the magnitude and composition of TILs in breast tumors are important for both response to therapy and improved prognosis.5-7High TILs prior to therapy has been demonstrated to be a biomarker of good prognosis in multiple large adjuvant and neoadjuvant breast cancer clinical trials, particularly in the more aggressive triple-negative (TN) and HER2-positive subtypes.5,6,8,9Understanding the baseline immune environment can lead to rational trial designs evaluating checkpoint inhibitor therapy and other combinations to benefit a wider subset of breast cancers.
The tumor immune environment is defined by the presence of cells from both the innate (including dendritic cells, macrophages, and neutrophils) and adaptive (B and T lymphocytes) immune system. The anti-tumor type 1 immunity develops through secretion of cytokines including tumor necrosis factor-alpha (TNF-a) and interferon gamma (IFNγ). The type 1 response is necessary for tumor elimination by triggering the tumor infiltrating antigen-presenting cells (APCs) to increase antigen presentation, releasing Type 1 CD4+helper T cells (Th1) that improve CD8+cytotoxic T-cell (CTL) function, and recruiting further type 1 immune response to the tumor.10However, breast cancer tumors typically develop a type 2 anti-inflammatory response.11The type 2 immune response promotes maintenance of proliferation signals, maintenance of angiogenesis, and decreased CD8+T-cell function through release of cytokines including IL-10 and IL-4. Type 2 CD4+helper T cells (Th2), including CD4+T cells that express the marker Forkhead box P3 (FOXP3), are important in suppressing the function of CTL and maintaining a type 2 tumor immune environment.12,13Therefore, in breast cancer, the type of immune response as well as the magnitude of immune response triggered is important in determining clinical outcome.
Lymphocyte Immune Infiltrate and Breast Cancer Prognosis
Increased lymphocytic infiltrate can be seen as early as benign ductal hyperplasia, increases in ductal carcinomain situ, and is highest in invasive breast cancer.14In patients with locally advanced breast cancer receiving neoadjuvant chemotherapy, tumors that have >50% lymphocytic infiltrate (lymphocyte predominant breast cancer or LPBC) are more likely to achieve a pathologic complete response (pCR) than patients with tumors with lower immune infiltrate. In a study of 1058 breast cancers, there was ~40% pCR in patients with LPBC tumors (odds ratio 1.38; 95% CI, 1.08-1.78;P=.012) while patients that had tumors with no lymphocytic infiltrate had ~7% pCR.5Increased CD8+T cells in the tumor have also been associated with improved survival, as demonstrated in a study of 1334 breast cancer patients (HR 0.55; 95% CI, 0.39-0.78;P= .001).7 However, the presence of FOXP3+T-cell infiltrate was associated with worse prognosis with patients who had high FOXP3+infiltrate (above the mean 15 cells/hpf) having decreased relapse-free survival (RFS) (HR 1.58; 95% CI, 1.01-2.47;P= .04) and decreased overall survival (OS) (HR 1.62; 95% CI, 0.96-2.74;P= .07) as compared with patients with tumors containing low FOXP3+.15The presence of lymphocytic infiltrate and particularly intratumoral type-1 T-cell infiltrate on clinical outcome differs considerably between breast cancer subtypes.
Effect of Tumor Immune Infiltrate Differs by Breast Cancer Subtype
The clinical benefit with even incremental increase in TILs has been most consistently demonstrated in TN breast cancer. In one study of 256 TN tumors, when TILs were evaluated as a continuous variable, each 10% increase in TIL correlated with a 17% increase in disease-free survival (DFS) (HR 0.83; 95% CI, 0.71-0.98;P= .023) and a 27% increase in OS (HR 0.73; 95% CI, 0.54-0.98;P= .035) and this finding has been confirmed in subsequent studies.6,9,16In the initial large adjuvant breast cancer studies that evaluated the benefit of increased TIL, only HER2-positive breast cancer patients that had had been treated with trastuzumab demonstrated improved prognosis with each 10% increase in TIL and was associated with decreased distant disease recurrence (n=209; HR 0.77; 95% CI, 0.61- 0.98;P= .02).17 However, one study has demonstrated in HER2-positive patients that for each 10% increase in stromal TIL there was 18% increase in OS (n=112; HR 0.82; 95% CI, 0.69-0.96).9 Although TN and HER2 breast cancer subtypes have seen clinical benefit with each 10% increase in TIL, HR-positive breast cancers have not shown this survival benefit even with LPBC. In one study evaluating 1078 HR-positive breast cancer patients, LPBC predicted neither improved DFS (HR 0.89; 95%CI, 0.44-1.8;P= .75) nor improved OS (HR 1.2; 95%CI, 0.53-2.7; P = .68).5,6
Evaluating type 1 T-cell infiltrate has also shown to predict improved clinical outcome in TN and HER2-positive breast cancer. In 927 TN breast cancer patients, CD8+T-cell infiltrate predicted improved disease-specific survival (DSS) (HR 0.35; 95% CI, 0.23-0.54;P= .001).18Presence of CD8+infiltrates has not been associated with prognostic benefit in HER2-positive breast cancer.15,18However, tumor infiltration of TBET+T cells (a Th1 CD4+T-cell type that supports CD8+T-cell proliferation) has been shown to predict improved RFS (n=102; HR 4.76; 95% CI, 1.07-20;P= .04) in HER2-positive tumors treated with trastuzumab.19Only one study has demonstrated improved breast cancer specific survival with CD8+T-cell infiltrate (n=2456; HR 0.91; 95% CI, 0.77-1.11;P= .046) in HR tumors and only 30% of HR tumors contained such infiltrates.18This has not been confirmed in other studies.17,20The presence of Th1 tumor infiltration predicts improved clinical outcomes in TN and HER2-positive breast cancer, but these clinical benefits are not consistently seen in HR-positive breast cancer.
When evaluating FOXP3+infiltrate as a marker for Th2 regulatory T-cells, both the TN and HER2-positive subtypes had tumors with high (greater than the median) FOXP3+infiltrate, but this did not predict worse outcome. However, HR-positive breast cancer had worse survival with high FOXP3+infiltrate. In 148 HR-positive tumors, high FOXP3+infiltrate was associated with a decreased RFS (HR 2.20; 95% CI, 1.26-3.85;P= .006) and OS (HR 2.57; 95% CI, 1.31-5.60,P= .006).15 Despite this breast cancer subtype not showing improved clinical outcome with increased TILs or Th1 immune infiltrate, the worse prognosis seen with FOXP3 infiltrate provides evidence of the importance of the tumor immune environment in the HR-positive subtype.
The differences in clinical outcome seen with tumor immune infiltrate between the breast cancer subtypes may be due to inherent differences between the subtypes. TN breast cancer has been found to have increased genetic mutations leading to aberrant proteins which the immune system sees as foreign, possibly increasing type 1 immune infiltrate.21,22Furthermore, TN tumors are associated with an increased B-cell metagene signature.23Increased antibody production from tumor-targeting B cells may stimulate antibody-dependent cellular cytotoxicity (ADCC) and natural killer (NK) T-cell tumor death.24In HER2-positive breast cancer, increased immune infiltrate has been associated with trastuzumab therapy. Trastuzumab has been shown to increase immune recognition of HER2-overexpressing tumors through ADCC and promote a Th1 tumor immune environment.25,26Regardless of cause, high tumor immune infiltrate in both TN and HER2-positive breast cancer indicates a subset of good prognosis tumors in otherwise aggressive, poor prognosis breast cancer subtypes. Unlike HER2-positive and TN tumors, HR-positive breast cancers have not shown the same clinical benefit with LPBC or type 1 T-cell infiltrate. One reason for this may be that expression of the estrogen receptor has been shown to promote a Th2 immune environment both by decreased expression of granzyme B and decreased MHC class II expression in breast cancer cells.27,28In HR-positive breast cancer, higher intratumoral FOXP3 infiltrate does predict worse outcome, therefore identifying methods to decrease regulatory T cells may improve HR breast cancer response to immune therapy. Systematic evaluation of pre-treatment TIL and understanding which specific subsets of tumor immune infiltrate influence prognosis in the different breast cancer subtypes may help guide how to use immune therapies and in the various combinations.29
Programmed death ligand 1 (PD-L1) expression has been associated with both poor prognosis30and improved prognosis in breast cancer.31,32PD-L1 expression is also associated with increased TIL infiltrate.32,33In 45 primary breast cancers, 89% of PD-L1-positive and 24% of PD-L1-negative tumors had moderate or diffuse TILs and 15% of PD-L1-negative breast cancers developed distant recurrence while no patients with PD-L1-positive breast cancer had recurrence.33In a study of 5,454 breast cancers, PD-L1 was most overexpressed in basal TN and HER2-positive breast cancer.34Furthermore, in TN breast cancers >5% cytoplasmic PD-L1 staining has been associated with improved OS in 161 patients (HR 0.45; 95%CI, 0.24-0.9;P= .035).32Several of the breast cancer trials evaluating PD-1 and PD-L1 inhibitors are requiring PD-L1 tumor positivity, but initial trial results have demonstrated, though the numbers are small, that some patients with negative PD-L1 still derive clinical benefit. Additional studies in breast cancer are needed to better understand the use of PD-L1 expression as a predictive marker of response to immune checkpoint blockade.
Clinical Trials of Checkpoint Inhibitors in Breast Cancer
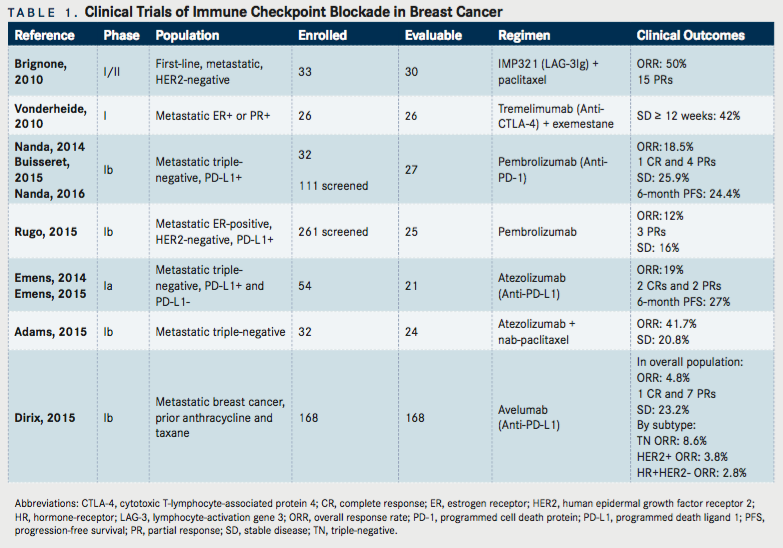
There are several immune-based therapeutic approaches that have been evaluated for breast cancer. These include cancer vaccines, cytokines, adoptive T-cell therapy, and immune checkpoint inhibitors. This review will focus only on agents or antibodies that block the immune checkpoints, including lymphocyte-activation gene 3 (LAG-3, CD223), cytotoxic T-lymphocyte-associated protein 4 (CTLA-4), programmed cell death protein 1 (PD-1) and PD-L1, that are undergoing clinical development for the treatment of breast cancer. The activity of immune checkpoint blockade has been reported in a small number of patients with metastatic breast cancer (TABLE 1). The majority of the reports are phase I studies reported in the form of abstracts.
LAG-3
LAG-3 is an inhibitory receptor that limits effector T-cell function and increases the suppressive activity of regulatory T cells. A published phase I/II trial (NCT00349934) evaluated IMP321, a recombinant soluble LAG-31g fusion protein given subcutaneously on Days 2 and 16, in combination with paclitaxel 80 mg/m2on Days 1, 8, and 15 of a 4-week cycle in 33 patients with metastatic HER2-negative breast cancer as first-line therapy.35This study showed an overall response rate (ORR) of 50%, with 15 partial responses. Six grade 3 adverse events were reported and included asthenia (3 cases), neutropenia, neuropathy, and an allergic reaction. Of note, treatment led to an induction of APCs, increased NK T cells, and increased effector-memory CD8+T cells. The combination of immune checkpoint inhibitors and chemotherapy represents a promising strategy that can potentiate adaptive anti-tumor immunity.
CTLA-4 antibodies
Ipilimumab and tremelimumab are monoclonal antibodies that target CTLA-4. Tremelimumab is a fully human monoclonal immunoglobulin (Ig) G2 antibody that blocks the binding of CTLA-4 to CD80 and CD86, resulting in enhanced T-cell activation.36Vonderheide et al evaluated tremelimumab, in combination with exemestane, a nonsteroidal aromatase inhibitor, in 26 postmenopausal women with metastatic HR-positive breast cancer that relapsed after previous treatment for metastatic disease.37Tremelimumab was administered at escalating doses intravenously once every 28 days or once every 90 days and exemestane orally at 25 mg daily. The majority of patients had received prior endocrine therapy, and five patients had prior exemestane. There were no objective responses, but 42% (11/26) of patients experienced stable disease for 12 weeks or more. The most frequently reported treatment-related adverse events (grades 1-3) included diarrhea (46%), pruritus (42%), constipation (23%), and fatigue (23%). Treatment was associated with increased peripheral CD4+and CD8+T-cells expression of inducible costimulatory (ICOS), and an increased ratio of ICOS+T cells to FOXP3+regulatory T cells in the peripheral blood, indicating T-cell activation. The recommended phase II dose was tremelimumab 6 mg/kg every 90 days in combination with exemestane, a lower dose than the 15 mg/kg every 90 days used in published phase II and III solid tumor trials.38,39Given the lack of objective anti-tumor activity in this setting, clinical trial efforts are now focused in evaluating tremelimumab in combination with radiation or PD-L1 inhibitors (TABLE 2).
There is limited experience with ipilimumab in breast cancer patients (NCT01502592). A single-dose of ipilimumab was tested in a window-of-opportunity trial in which breast cancer patients with a tumor measuring ≥1.5 cm were randomized to intratumoral cryoablation, preoperative ipilimumab, or intratumoral cryoablation and preoperative ipilimumab. The outcome measures were safety, tolerability, and immunologic response pre- and post-treatment. Early results from this trial show cryoablation and ipilimumab increased type 1 anti-tumor immune response both with increased effector T cell to regulatory T-cell ratio in the tumor and increased peripheral activated T-cell populations as compared to either cryoablation or ipilimumab alone.40
PD-1 Inhibitors
Pembrolizumab (MK-3475) is a fully human IgG4 monoclonal antibody that directly blocks the interaction between the T-cell inhibitory molecule PD-1 and its ligands, PD-L1 and PD-L2. Several multi-cohorts, non-randomized Phase Ib basket trials have been conducted of pembrolizumab in different tumor or histological types within one study. In a multi-institution phase Ib trial (KEYNOTE-012, NCT01848834), 111 metastatic TN breast cancer were screened for PD-L1 expression, and 65 (59%) met criteria for being PD-L1 positive, which was defined as staining in the stroma or in ≥ 1% of tumor cells assessed by an immunohistochemistry assay using a 22C3 antihuman PD-1 antibody.41-43In this study, 32 patients received pembrolizumab 10 mg/kg intravenously every 2 weeks. About 47% of patients received three or more lines of prior therapy for metastatic disease, and all had prior taxane exposure. The ORR based on central review was 18.5% in 27 breast cancer patients evaluable for response. Median time to response was 17.9 weeks (range, 7.3-32.4). One complete response occurred, four partial responses were observed, and 25.9% (n=7/27) had stable disease. Additionally, the 6-month progression-free survival (PFS) rate was 23.3%. Immune-mediated adverse events included one grade 2 hypothyroidism, one grade 3 hepatitis, and one grade 3 colitis.
Another phase Ib trial (KEYNOTE-028; NCT02054806) screened 261 metastatic ER-positive, HER2-negative breast cancer patients for PD-L1 positivity using the same definition and assay as above.44Out of 248 evaluable samples, 19.4% were PD-L1 positive. About 16% of patients received three or more lines of prior therapy for metastatic disease. The ORR based on investigator radiologic review in the 25 patients that were evaluable was 12% (95% CI, 2.5-31.2); 3 of 25 patients had a partial response. In addition, 16% (4/23) experienced stable disease. Immune-mediated adverse events included hepatitis (grade 3, 4%), hypothyroidism (grade 1 and 2, 16%), and hyperthyroidism (grade 2, 4%). Although these trials treated a small number of patients, they demonstrate that single-agent pembrolizumab is tolerated and has activity in a subset of pretreated metastatic TN and ER-positive, HER2-negative breast cancer patients. Interestingly, responses were not uniform with some patients not responding, despite their tumors expressing PD-L1. The predictive value of PD-L1 expression needs further validation.
A phase III study (KEYNOTE-119; NCT02555657) randomizing patients with metastatic TN breast cancer who have received no more than two prior systemic treatments for metastatic disease to pembrolizumab or chemotherapy chosen by the treating physician, and consisting of either capecitabine, eribulin, gemcitabine, or vinorelbine, is currently underway. There is central determination of PD-L1 tumor status. The primary outcome measures are PFS and OS, and the accrual goal is 600 patients. There are several other ongoing clinical trials of pembrolizumab as monotherapy or in combination with different chemotherapy agents, endocrine therapy, or with radiation. Other studies are combining pembrolizumab with targeted drugs such as trastuzumab or a poly(ADP-ribose) polymerase (PARP) inhibitor (TABLE 1). The results of such trials will help further the clinical development of immunotherapy in the different breast cancer subtypes.
Nivolumab (BMS-936558) is a fully human monoclonal IgG4 antibody that also targets PD-1. There are several studies ongoing to evaluate nivolumab with different chemotherapy agents or other targeted agents in the treatment of metastatic breast cancer (TABLE 2).
PD-L1 Inhibitors
Atezolizumab (MPDL3280A) is an engineered humanized IgG1 monoclonal antibody that targets human PD-L1 and inhibits its interaction with its receptors, PD-1 and B7.1 (CD80), which generate inhibitory signals to T cells. Because PD-L1 is expressed on activated T cells, atezolizumab was engineered with a modified Fc domain, eliminating ADCC to prevent a reduction in the T cells expressing PD-L1. The safety and ef - cacy of atezolizumab were assessed in a phase I study that enrolled patients with metastatic solid tumors (NCT01375842). Preliminary results in the metastatic TN breast cancer cohort were presented at the 2014 San Antonio Breast Cancer Symposium.45Updated results were presented at the 2015 Annual Meeting of the American Association of Cancer Research.46The trial enrolled a cohort of 54 metastatic TN breast cancer patients, with both PD-L1positive and PD-L1–negative tumors. Atezolizumab was administered intravenously at 15 mg/kg, 20 mg/kg or fixed dose of 1200 mg every 3 weeks. PD-L1 expression was assessed immunohistochemically on tumor-infiltrating immune cells using a proprietary SP142 assay. PD-L1 positivity was defined as IHC3 (≥10% PD-L1 positive) or IHC2 (≥5% but <10%). The majority of patients (85%) had received four or more systemic regimens, including adjuvant, neoadjuvant, and metastatic treatments. About 69% of these patients had PD-L1–positive (IHC 2/3) disease. Twenty-one patients were evaluable for efficacy, and their tumors were PD-L1-positive (IHC 2/3). The ORR was 19% (95% CI, 5-42%), including two complete responses and two partial responses. Notably, 3 patients experienced pseudo-progression and then went on to have tumor shrinkage. The 24-week PFS was 27% (95% CI, 7-47%). The most common all grade adverse events were fatigue (22%), fever (15%), neutropenia (15%), and nausea (15%). A single patient with an atrial septal defect had a grade 5 adverse event of pulmonary hypertension. Studies show that chemotherapy has the capacity to potentially augment cancer immunity by decreasing tumor burden, and generating immunogenic cell death.47Data from an ongoing phase Ib study of atezolizumab in combination with nab-paclitaxel in metastatic TN breast cancer was presented at the 2015 San Antonio Breast Cancer Symposium (NCT01633970).48
The use of nab-paclitaxel is appealing to combine with immunotherapy as it does not require steroid premedication, which can cause immunosuppression. PD-L1 expression was assessed on tumor-infiltrating immune cells with the Ventana SP142 immunohistochemistry assay. PD-L1 expression in the tumor-infiltrating immune cells was scored as 0, 1, 2, or 3 if <1%, ≥ 1% and <5%, ≥5% and <10%, or ≥10%, respectively. Thirty-two patients were treated with atezolizumab 800 mg IV on days 1 and 15 with nab-paclitaxel 125 mg/m2IV on days 1, 8, and 15 on a 28-day cycle. The median number of prior regimens was 5 (range, 1-10). The confirmed ORR in 24 patients evaluable for response was 41.7% (95% CI, 22.1-63.4%), which included a complete response rate of 4.2% and a partial response rate of 66.7%. Disease stabilization was achieved in 20.8% of patients. In the first-line setting, the confirmed ORR in 9 patients was 66.7% (95% CI, 29.9-92.5). In the second-line setting, the confirmed ORR in 8 patients was 25% (95% CI, 3.2-65.1). The ORR by PD-L1 expression in the tumor-infiltrating immune cells scored as 0, 1-3, and unknown were 57.1% (95% CI, 18.4-90.1), 77.8% (95% CI, 40-97.2), and 75% (95%, 34.9-96.8), respectively. At the time of analysis, 11 of 17 responses continued. The reported treatment-related adverse events were grade 3-4 neutropenia (41%), grade 3-4 thrombocytopenia (9%), and grade 3-4 anemia (6%). Interestingly, responses were still observed in TN breast tumors lacking expression of PD-L1, although higher in the tumors that expressed PD-L1. Though not powered to show a difference, the response rate was higher in patients who received the combination as first-line therapy compared to the group that had already received prior lines of therapy.
These encouraging results formed the basis to initiate a larger trial effort in metastatic TN breast cancer in the first-line setting. IMpassion130 (NCT02425891) is a phase III randomized (1:1) placebo-controlled trial of atezolizumab in combination with nab-paclitaxel compared with placebo with nab-paclitaxel for patients with previously untreated metastatic TN breast cancer, currently underway.49Atezolizumab 840 mg is administered intravenously on Days 1 and 15 and nab-paclitaxel 100 mg/m2is given intravenously on Days 1, 8, and 15 of a 28-day cycle. The primary endpoint is PFS and the accrual goal is 900 patients.
Early results from a phase Ib solid tumor trial (JAVELIN; NCT01772004) were presented at the 2015 San Antonio Breast Cancer Symposium evaluating avelumab (MSB0010718C), a fully human anti-PD-L1 IgG1 antibody in 168 patients with metastatic breast cancer (all subtypes) who had received prior anthracycline and taxane, regardless of PD-L1 status.50This report contains the largest number of breast cancer patients to date treated with a checkpoint inhibitor. Avelumab was given at 10 mg/kg intravenously every 2 weeks until progression. By subtype, 42.9% (n=72) were HR-positive and HER2-negative, 34.5% (n=58) were TN, 15.5% (n=26) were HER2-positive, and 7.1% (n=12) were unknown. The majority of patients (52.4%) had received three or more prior regimens for metastatic disease. Avelumab was administered at 10 mg/kg intravenously every 2 weeks. In the overall population, which included PD-L1-negative patients, the ORR was 4.8% (95% CI, 2.1-9.2); one complete response and seven partial responses. Disease stabilization occurred in 23.2% of patients. The median duration of response was 28.7 weeks. By subtype, ORR was 8.6% (95% CI, 2.9-19) in PD-L1-positive TN breast cancer with 5 partial responses, 3.8% in HER2-positive (95% CI, 0.1-19.6), and 2.8% (95% CI, 0.3-9.7) in HR-positive, HER2-negative. Preliminary results in the TN breast cancer subset with PD-L1-positive tumor infiltrate revealed a higher response rate of 44.4% (4/9) compared with 2.6% in the patients with PD-L1-negative tumor infiltrate (1/39). According to PD-L1 expression level in 136 evaluable patients, ORR was 33.3% (4/12) with high PD-L1 expression within the tumor compared to 2.4% (3/124) with low PD-L1 expressing immune cells. Prominent immune-related, treatment-related adverse events included hypothyroidism (grade 1-2, 4.8%), hepatitis (grade 3, 1.8%), and pneumonitis (grade 1-3, 1.8%).
The results suggest that in an unselected cohort of metastatic breast cancer, antitumor activity of a PD-L1 inhibitor is low, but specific subsets, such as the TN, experienced clinical benefit. PD-L1 expression in tumor infiltrate in TN breast cancer appears to be associated with response to avelumab. In this particular study, the benefit of checkpoint inhibitor therapy in breast cancer has emerged with evaluating the breast cancer subtypes separately. Further correlation of PD-L1 expression as a predictive marker of response to checkpoint inhibitors is needed.
Durvalumab (MEDI 4736) is another human monoclonal antibody of the IgG1 kappa subclass that interferes with the interactions between PD-L1 and PD-1 and B7.1, molecules that are expressed on antigen-presenting cells and T-cells. Several studies are ongoing or will be accruing in early stage and advanced breast cancer evaluating durvalumab monotherapy, durvalumab in combination with chemotherapy or other targeted therapy (TABLE 2).
Conclusions
Standardizing how to characterize the breast tumor immune environment will allow identification of patients that may only need therapy with checkpoint inhibitors, but also identify patients with lower immune infiltrate that may benefit from a combination of checkpoint blockade with other therapies, broadening the range of benefit in breast cancer patients. The small phase I studies described to date show activity of checkpoint inhibitors in a subset of metastatic breast cancer patients with responses that appear durable. The results from the phase II and III trials are eagerly awaited to confirm the benefits of immunotherapy in breast cancer. The hope is that these immunotherapy agents will soon be included in our breast cancer treatment paradigms.
References
- Pages F, Kirilovsky A, Mlecnik B, et al. In situ cytotoxic and memory T cells predict outcome in patients with early-stage colorectal cancer.J Clin Oncol. 2009;27(35):5944-5951.
- Hwang WT, Adams SF, Tahirovic E, Hagemann IS, Coukos G. Prognostic significance of tumor-infiltrating T cells in ovarian cancer: a meta-analysis.Gynecol Oncol. 2012;124(2):192-198.
- Dieu-Nosjean MC, Antoine M, Danel C, et al. Long-term survival for patients with non-small-cell lung cancer with intratumoral lymphoid structures.J Clin Oncol. 2008;26(27):4410-4417.
- Azimi F, Scolyer RA, Rumcheva P, et al. Tumor-infiltrating lymphocyte grade is an independent predictor of sentinel lymph node status and survival in patients with cutaneous melanoma.J Clin Oncol. 2012;30(21):2678-2683.
- Denkert C, Loibl S, Noske A, et al. Tumor-associated lymphocytes as an independent predictor of response to neoadjuvant chemotherapy in breast cancer.J Clin Oncol. 2010;28(1):105-113.
- Loi S, Sirtaine N, Piette F, et al. Prognostic and predictive value of tumor- infiltrating lymphocytes in a phase III randomized adjuvant breast cancer trial in node-positive breast cancer comparing the addition of docetaxel to doxorubicin with doxorubicin-based chemotherapy: BIG 02-98.J Clin Oncol. 2013;31(7):860- 867.
- Mahmoud SM, Paish EC, Powe DG, et al. Tumor-infiltrating CD8+ lymphocytes predict clinical outcome in breast cancer.J Clin Oncol. 2011;29(15):1949-1955.
- Denkert C, von Minckwitz G, Brase JC, et al. Tumor-infiltrating lymphocytes and response to neoadjuvant chemotherapy with or without carboplatin in human epidermal growth factor receptor 2-positive and triple-negative primary breast cancers.J Clin Oncol. 2015;33(9):983-991.
- Dieci MV, Mathieu MC, Guarneri V, et al. Prognostic and predictive value of tumor- infiltrating lymphocytes in two phase III randomized adjuvant breast cancer trials.Ann Oncol. 2015;26(8):1698-1704.
- Zitvogel L, Galluzzi L, Kepp O, Smyth MJ, Kroemer G. Type I interferons in anticancer immunity.Nat Rev Immunol. 2015;15(7):405-414.
- Pedroza-Gonzalez A, Xu K, Wu TC, et al. Thymic stromal lymphopoietin fosters human breast tumor growth by promoting type 2 inflammation.J Exp Med. 2011;208(3):479-490.
- Coussens LM, Zitvogel L, Palucka AK. Neutralizing tumor-promoting chronic inflammation: a magic bullet?Science. 2013;339(6117):286-291.
- Tan AH, Goh SY, Wong SC, Lam KP. T helper cell-specific regulation of inducible costimulator expression via distinct mechanisms mediated by T-bet and GATA-3.J Biol Chem. 2008;283(1):128-136.
- Hussein MR, Hassan HI. Analysis of the mononuclear inflammatory cell infiltrate in the normal breast, benign proliferative breast disease, in situ and infiltrating ductal breast carcinomas: preliminary observations.J Clin Pathol. 2006;59(9):972- 977.
- Bates GJ, Fox SB, Han C, et al. Quantification of regulatory T cells enables the identification of high-risk breast cancer patients and those at risk of late relapse.J Clin Oncol. 2006;24(34):5373-5380.
- Adams S, Gray RJ, Demaria S, et al. Prognostic value of tumor-infiltrating lymphocytes in triple-negative breast cancers from two phase III randomized adjuvant breast cancer trials: ECOG 2197 and ECOG 1199.J Clin Oncol. 2014;32(27):2959-2966.
- Loi S, Michiels S, Salgado R, et al. Tumor infiltrating lymphocytes is prognostic and predictive for trastuzumab benefit in early breast cancer: results from the FinHER trial.Ann Oncol. 2014;25:1544-1550.
- Liu S, Lachapelle J, Leung S, Gao D, Foulkes WD, Nielsen TO. CD8+ lymphocyte infiltration is an independent favorable prognostic indicator in basal-like breast cancer.Breast Cancer Res. 2012;14(2):R48.
- Ladoire S, Arnould L, Mignot G, et al. T-bet expression in intratumoral lymphoid structures after neoadjuvant trastuzumab plus docetaxel for HER2-overexpressing breast carcinoma predicts survival.Br J Cancer. 2011;105(3):366-371.
- Baker K, Lachapelle J, Zlobec I, Bismar TA, Terracciano L, Foulkes WD. Prognostic significance of CD8+ T lymphocytes in breast cancer depends upon both oestrogen receptor status and histological grade.Histopathology. 2011;58(7):1107-1116.
- Smid M, Hoes M, Sieuwerts AM, et al. Patterns and incidence of chromosomal instability and their prognostic relevance in breast cancer subtypes.Breast Cancer Res Treat. 2011;128(1):23-30.
- Disis ML, Stanton SE. Triple-negative breast cancer: immune modulation as the new treatment paradigm.Am Soc Clin Oncol Educ Book. 2015:e25-30.
- Rody A, Karn T, Liedtke C, et al. A clinically relevant gene signature in triple negative and basal-like breast cancer.Breast Cancer Res. 2011;13(5):R97.
- Ferris RL, Jaffee EM, Ferrone S. Tumor antigen-targeted, monoclonal antibody-based immunotherapy: clinical response, cellular immunity, and immunoescape.J Clin Oncol. 2010;28(28):4390-4399.
- Park S, Jiang Z, Mortenson ED, et al. The therapeutic effect of anti-HER2/neu antibody depends on both innate and adaptive immunity.Cancer Cell. 2010;18(2):160-170.
- Arnould L, Gelly M, Penault-Llorca F, et al. Trastuzumab-based treatment of HER2-positive breast cancer: an antibody-dependent cellular cytotoxicity mechanism?British Journal of Cancer. 2006;94(2):259-267.
- Jiang X, Ellison SJ, Alarid ET, Shapiro DJ. Interplay between the levels of estrogen and estrogen receptor controls the level of the granzyme inhibitor, proteinase inhibitor 9 and susceptibility to immune surveillance by natural killer cells.Oncogene. 2007;26(28):4106-4114.
- Mostafa AA, Codner D, Hirasawa K, et al. Activation of ERalpha signaling differentially modulates IFN-gamma induced HLA-class II expression in breast cancer cells.PLoS One. 2014;9(1):e87377.
- Salgado R, Denkert C, Demaria S, et al. The evaluation of tumor-infiltrating lymphocytes (TILs) in breast cancer: recommendations by an International TILs Working Group 2014.Ann Oncol. 2015;26(2):259-271.
- Muenst S, Schaerli AR, Gao F, et al. Expression of programmed death ligand 1 (PD-L1) is associated with poor prognosis in human breast cancer.Breast Cancer Res Treat. 2014;146(1):15-24.
- Schalper KA, Velcheti V, Carvajal D, et al. In situ tumor PD-L1 mRNA expression is associated with increased TILs and better outcome in breast carcinomas.Clin Cancer Res. 2014;20(10):2773-2782.
- Beckers RK, Selinger CI, Vilain R, et al. PDL1 expression in triple-negative breast cancer is associated with tumour-infiltrating lymphocytes and improved outcome.Histopathology. 2015 Epub ahead of print.
- Cimino-Mathews A, Thompson E, Taube JM, et al. PD-L1(B7-H1)expression and the immune tumor microenvironment in primary and metastatic breast carcinomas.Hum Pathol. 2016;47(1):52-63.
- Sabatier R, Finetti P, Mamessier E, et al. Prognostic and predictive value of PDL1 expression in breast cancer.Oncotarget. 2015;6(7):5449-5464.
- Brignone C, Gutierrez M, Mefti F, et al. First-line chemoimmunotherapy in metastatic breast carcinoma: combination of paclitaxel and IMP321 (LAG-3Ig) enhances immune responses and antitumor activity.J Transl Med. 2010;8:71.
- Tarhini AA, Kirkwood JM. Tremelimumab(CP-675,206):a fully human anticytotoxic T lymphocyte-associated antigen 4 monoclonal antibody for treatment of patients with advanced cancers.Expert Opin Biol Ther. 2008;8(10):1583-1593.
- Vonderheide RH, LoRusso PM, Khalil M, et al. Tremelimumab in combination with exemestane in patients with advanced breast cancer and treatment-associated modulation of inducible costimulator expression on patient T cells.Clin Cancer Res. 2010;16(13):3485-3494.
- Ribas A, Kefford R, Marshall MA, et al. Phase III randomized clinical trial comparing tremelimumab with standard-of-care chemotherapy in patients with advanced melanoma.J Clin Oncol. 2013;31(5):616-622.
- Calabro L, Morra A, Fonsatti E, et al. Efficacy and safety of an intensified schedule of tremelimumab for chemotherapy-resistant malignant mesothelioma: an open-label, single-arm, phase 2 study.Lancet Respir Med. 2015;3(4):301-309.
- Page DB, Diab A, Yuan J, et al. Pre-operative immunotherapy with tumor cryoablation plus ipilimumab induces potentially favorable systemic and intratumoral immune effects in early stage breast cancer patients.J Immunother Cancer. 2015;3(Suppl 1):O6. doi:10.1186/2051-1426-3-S1-O6.
- Nanda R, Chow LQ, Dees EC, et al. A phase Ib study of pembrolizumab (MK-3475) in patients with advanced triple-negative breast cancer [abstract]. In: Proceedings of the Thirty-Seventh Annual CTRC-AACR San Antonio Breast Cancer Symposium; 2014 Dec 913; San Antonio, TX. Philadelphia (PA):American Association for Cancer Research;Cancer Res2015;75(9 Suppl):Abstract nr S109.
- Buisseret L, Specht J, Dees EC, et al: KEYNOTE-012: A phase Ib study of pembrolizumab (MK-3475) in patients with metastatic triple-negative breast cancer.Ann Oncol. 2015; 26 (suppl 3): abstr 14P.
- Nanda R, Chow Q, Dees C, et al. Pembrolizumab in patients with advanced triple-negative breast cancer: Phase Ib KEYNOTE-012 study.J Clin Oncol. 2016:Published online on May 2, 2016.
- Rugo HS, Delord J-P, Im S-A, et al. Preliminary efficacy and safety of pembrolizumab (MK-3475) in patients with PD-L1-positive, estrogen receptor-positive (ER+)/HER2-negative advanced breast cancer enrolled in KEYNOTE-028 [abstract]. In: Proceedings of the Thirty-Eighth Annual CTRC-AACR San Antonio Breast Cancer Symposium; 2015 Dec 610; San Antonio, TX. Philadelphia (PA): American Association for Cancer Research; 2015. Abstract nr S5–07.
- Emens LA, Braiteh FS, Cassier P, et al. Inhibition of PD-L1 by MPDL3280A leads to clinical activity in patients with metastatic triple-negative breast cancer [abstract]. In: 2014 San Antonio Breast Cancer Symposium: San Antonio, TX, 2014, December 8-13, 2014.
- Emens LA, Braiteh FS, Cassier P, et al. Inhibition of PD-L1 by MPDL3280A leads to clinical activity in patients with metastatic triple-negative breast cancer [abstract]. In: Proceedings of the 106th Annual Meeting of the American Association for Cancer Research; 2015 Apr 1822; Philadelphia, PA. Philadelphia (PA): AACR;Cancer Res2015;75(15 Suppl):Abstract nr 2859.
- Chen G, Emens LA. Chemoimmunotherapy: reengineering tumor immunity.Cancer Immunol Immunother. 2013;62(2):203-216.
- Adams S, Diamond J, Hamilton E, et al. Safety and clinical activity of atezolizumab (anti-PDL1) in combination with nab-paclitaxel in patients with metastatic triple-negative breast cancer [abstract]. In: Proceedings of the Thirty-Eighth Annual CTRC-AACR San Antonio Breast Cancer Symposium; 2015 Dec 610; San Antonio, TX. Philadelphia (PA): American Association for Cancer Research; 2015. Abstract nr P2-11-06.
- Emens L, Adams S, Loi S, et al: A phase III randomized trial of atezolizumab in combination with nab-paclitaxel as first line therapy for patients with metastatic triple-negative breast cancer.Cancer Res2016;76(4 Suppl): abstr OT1-01-06.
- Dirix LY, Takacs I, Nikolinakos P, et al. Avelumab (MSB0010718C), an anti-PD-L1 antibody, in patients with locally advanced or metastatic breast cancer: a phase Ib JAVELIN solid tumor trial [abstract]. In: Proceedings of the Thirty-Eighth Annual CTRC-AACR San Antonio Breast Cancer Symposium; 2015 Dec 610; San Antonio, TX. Philadelphia (PA): American Association for Cancer Research; 2015. Abstract nr S1–04.

Batalini Explores Role of UGT1A1 in Patients Treated With Sacituzumab Govitecan for HR+ MBC
April 22nd 2024During a Community Case Forum live event in partnership with The Arizona Clinical Oncology Society, Felipe Batalini, MD, discussed the TROPiCS-02 trial of sacituzumab govitecan and the impact of the UGT1A1 status on adverse event frequency.
Read More
Novel Approaches Focus on Limiting Toxicity in Older Patients With ALL
April 22nd 2024The major challenges for clinicians treating older patients with acute lymphoblastic leukemia surround the emergence of resistance to existing therapies and the toxicities associated with current chemotherapies.
Read More