Broader Applications for Targeted Agents in Breast Cancer Expand Patient Use
A greater understanding of the mechanisms underlying endocrine resistance, along with the development of targeted agents directed at key regulatory oncogenic pathways, continue to lead to new options in the treatment of hormone receptor–positive, HER2-negative breast cancer. These therapies offer the promise of better disease control rates and improved quality of life for patients with advanced disease.
Joshua J. Gruber, MD

Joshua J. Gruber, MD
A greater understanding of the mechanisms underlying endocrine resistance, along with the development of targeted agents directed at key regulatory oncogenic pathways, continue to lead to new options in the treatment of hormone receptor (HR)positive, HER2-negative breast cancer. These therapies offer the promise of better disease control rates and improved quality of life for patients with advanced disease.1
HR-positive, HER2-negative breast cancer is the most common subtype, accounting for more than 70% of breast cancer cases.2Endocrine therapy is the mainstay of first-line treatment of this disease in patients without visceral crises, yielding improved outcomes in early and metastatic disease.3However, de novo or acquired resistance to endocrine therapy remains a significant clinical challenge, leading to therapeutic failure and limiting survival, especially in patients with metastatic disease.3
Targeted agents approved or in clinical development for treatment of HR-positive, HER2-negative disease include CDK4/6 inhibitors, selective PI3K inhibitors, and PARP inhibitors. These advances have had practice-changing implications; for instance, CDK4/6 inhibitors have yielded significant improvements in progression-free survival (PFS) and are now the treatment of choice in patients with HR- positive, HER2-negative, treatment-naïve or endocrine therapypretreated metastatic breast cancer without visceral crisis.1In addition, exciting overall survival (OS) data from pivotal trials for CDK4/6 inhibitors are helping to inform the magnitude of benefit in patients with advanced ormetastatic disease.4-6
“The value of having these different inhibitors on the market is in the ability to tailor [treatment] to a specific patient who may have certain comorbidities and be able to give them the appropriate therapy,” said Joshua J. Gruber, MD, an instructor of medicine in the division of medical oncology at Stanford University Medical Center in California.
Moreover, alpelisib (Piqray), the first FDA-approved PI3K inhibitor for the treatment of postmenopausal women, as well as men, withPIK3CA-mutated tumors, also gained the first new drug application for a new molecular entity approved under the FDA’s Real-Time Oncology Review pilot program.7
In Recent Updates, CDK4/6 inhibitors Show Survival Advantages
Three CDK4/6 inhibitors are approved for breast cancer. The first, palbociclib (Ibrance), received accelerated approval in 2015 for the first-line treatment of estrogen receptor (ER)positive, HER2-negative advanced breast cancer for postmenopausal women, in combination with letrozole, based on the phase II PALOMA-1 study.8 Later, both ribociclib (Kisqali) and abemaciclib (Verzenio) gained similar indications in this patient population based on study results in MONALEESA-29,10and MONARCH 3,11,12respectively. Additionally, abemaciclib is approved as monotherapy for adults who progressed following endocrine therapy and prior chemotherapy in the metastatic setting.
Ribobiclib
Results of a protocol-specified interim analysis of OS from ribociclib treatment in the MONALEESA-7 trial were recently published and showed an OS rate at 42 months of 70.2% (95% CI, 63.5%-76.0%) in the ribociclib group compared with 46.0% (95% CI, 32.0%-
58.9%) in the placebo group (hazard ratio, 0.71; 95% CI, 0.54-0.95; P = .00973 by log-rank test).4,5
“Improvement in overall survival is the best endpoint one could hope for,” said Aditya Bardia, MD, MPH, one of the report’s authors, as well as an assistant professor of medicine at Harvard Medical School and an attending physician at Massachusetts General Hospital, both in Boston. “[This] highlights the impact of CDK4/6 inhibitors in changing the natural history of metastatic HR-positive breast cancer.” Results of the MONALEESA-3 trial presented at the European Society for Medical Oncology (ESMO) 2019 Congress showed that first and second-line treatment with the CDK4/6 inhibitor ribociclib plus fulvestrant significantly improved overall survival in postmenopausal patients with HR-positive, HER2-advanced breast cancer. These benefits were seen in women not previously treated with hormonal therapy as well as in those who had become resistant to endocrine therapy.13
“[CDK4/6 inhibitors] seem to improve survival by about a year in patients with ER-positive disease,” Gruber said. “It has been one of the most important advancements in metastatic breast cancer within the past 5 or 10 years. It has had a dramatic impact.”
Previously reported data from MONALEESA-3 showed that ribociclib induced a median PFS of 20.5 months (95% CI, 18.5-23.5) versus placebo at 12.8 months (95% CI, 10.9-16.3), resulting in a statistically significant improvement in the primary endpoint (hazard ratio, 0.593;95% CL, 0.480-0.732,P<.001).14
In a recent reversal of an earlier decision to reject ribociclib, the National Institute of Health and Care Excellence in the United Kingdom recommended ribociclib in combination with fulvestrant for use on the British National Health Servicewhere exemestane plus everolimus is the most appropriate alternative—based on the second-line subpopulation of the MONALEESA-3 trial. In this subgroup of patients who had received up to 1 line of prior endocrine therapy for advanced disease, the ribociclib combination demonstrated a median PFS of 14.6 months compared with 9.1 months for the placebo arm.14,15
Abemaciclib
Another CDK4/6 inhibitor, abemaciclib is highly selective, reversible, and currently the most potent of the approved agents, with reported concentration of inhibitor required for achieving 50% inhibition, or IC50, of 2 nM and 10 nM for CDK4 and CDK6, respectively. The brain-blood-barrier crossing potential of abemaciclib has also generated interest in clinical evaluation in patients with ER-positive breast cancer and brain metastases.16
Assessment of abemaciclib levels from clinical samples obtained in the JPBO studya phase II, Simon 2-stage design trial evaluating 6 patient cohorts with brain metastases secondary to HR-positive metastatic breast cancer, nonsmall cell lung cancer, or melanoma—showed comparable abemaciclib levels in brain metastases tissues, cerebrospinal fluid, and time-matched plasma samples from patients with brain metastases secondary to HR-positive metastatic breast cancer.17
In data presented at the 2019 American Society of Clinical Oncology (ASCO) Annual Meeting, analyses of outcomes in part 2 of the study showed that abemaciclib monotherapy yielded a confirmed objective intracranial response rate (OIRR) of 6% in 52 evaluable patients. The intracranial clinical benefit rate, which included all responses of stable disease lasting 6 months or better, was 25% and the median PFS was 4.4 months (95% CI, 2.6-5.5). Although the study did not meet the primary endpoint of 11% OIRR, the intracranial benefit in this heavily pretreated population of patients with poor prognoses prompted the authors to conclude that further studies of abemaciclib monotherapy and combinations for controlling brain metastases in cancers, including in advanced breast cancer, are warranted.18
Also at 2019 ESMO Congress, MONARCH-2 showed statistically and clinically meaningful improvement in overall survival with the CDK4/6 inhibitor abemaciclib plus fulvestrant in pre-, and peri-as well as in postmenopausal women patients with HR-positive, HER2- advanced breast cancer resistant to hormonal therapy.19The abemaciclib-plus-fulvestrant combination was approved based on PFS data from MONARCH 2, in which the combination significantly extended PFS compared with fulvestrant alone (median, 16.4 vs 9.3 months; hazard ratio, 0.553;95% Cl, 0.449-0.681;P<.001).20
“It would be important to see the published data,” Bardia said, when asked how this relates to the OS findings observed with ribociclib therapy. “[Although] the CDK4/6 inhibitors are broadly similar, there are subtle differences in potency; ability to impact CDK 4, CDK 6, and other CDKs; and safety profile. The latter in particular tends to [affect] clinical decision making in the setting of similar efficacy (TABLE).9,11”
Findings were presented in a Presidential Symposium at the ESMO 2019 Congress.6
PI3K Inhibitors forPIK3CA-Mutated Disease
Activating mutations or amplifications of thePIK3CAgene that encodes p110α dysregulate PI3Kα expression and are common in many cancers, including breast cancer.21Clinical development of PI3K inhibitors is predicated on the importance of PI3K/AKT signaling in tumor growth and proliferation promotion, high frequency ofPIK3CAmutations in HR-positive breast cancer, and their association with the luminal B breast cancer subtype, as well as unfavorable OS outcomes in node-positive disease.22-24
Agents Under Investigation
The pan-PI3K inhibitor buparlisib and the PI3K-selective inhibitors taselisib and alpelisib have been tested in clinical trials, and the FDA recently granted an approval to alpelisib combined with fulvestrant for the treatment of postmenopausal women, as well as men, withPIK3CA-mutated tumors.7This marks the entry of the first PI3K inhibitor into the breast cancer therapeutic landscape.
Taselib
The potent and selective PI3K inhibitor taselisib (GDC-0032) has demonstrated greater sensitivity for mutant PI3Kα isoforms than wild-type PI3Kα, and preclinical studies have demonstrated the agent’s potent proliferation inhibition activity on p110α-mutant breast cancer cell lines (IC50, 70 nM), as well as tumor growth inhibition in human breast cancer xenograft models harboringPIK3CAmutations.25
In June 2018, the company responsible for developing the drug decided not to pursue an FDA submission based on data from the phase III SANDPIPER trial, which showed that taselisib and fulvestrant improved PFS by a modest 2 months in patients with HR-positive, HER2-negative breast cancer but resulted in more toxicity.26,27
“[These] findings are proof that targeting this pathway in breast cancer is effective; however, the benefits to patients are more modest than we had hoped for,” said José Baselga, MD, PhD, the study’s lead author, who presented the SANDPIP- ER data at the 2018 ASCO Annual Meeting.27Still optimistic about the potential role of this class of agents, Bardia said that results of trials testing taselisib in this patient population “[highlight] the therapeutic role of PI3K inhibitionfor PIK3CA-mutant tumors, but also the need to carefully weigh toxicity considerations.”
Alpelsib
An orally bioavailable PI3Kα-selective inhibitor, alpelisib has demonstrated higher efficacy (IC50, about 4 nM) and wider safety profile than the pan-class I PI3K inhibitor buparlisib in patients with PIK3CAmutations.28
The alpelisib approval was based on data from the phase III SOLAR-1 trial, which evaluated fulvestrant with either alpelisib or placebo in patients with HR-positive, HER2-negative breast cancer who received prior treatment with endocrine therapy.7 In the cohort of patients withPIK3CA-mutated cancer (n = 341), PFS was 11.0 months (95% CI, 7.5-14.5) in the alpelisib group compared with 5.7 months (95% CI, 3.7-7.4) in the placebo group (hazard ratio, 0.65; 95% CI, 0.50-0.85;P<.001).29
The overall response rate among all the patients in the cohort withPIK3CA-mutated breast cancer was greater with alpelisib at 26.6% versus placebo at 12.8%.
Concomitant with the approval of alpelisib, the FDA approved the companion diagnostic test therascreen PIK3CA RGQ PCR Kit (Qiagen) to select patients who havePIK3CAmutations.7In their review of recent advancements in the treatment of metastatic breast cancer, the authors led by Marie-France Savard, MD, said, “The results of SOLAR-1 represented a notable milestone in precision medicine and for the use of PI3K inhibitors in metastatic breast cancer.”30
PARP Inhibitors
The synthetic lethality invoked by PARP inhibition in the background ofBRCA1/2mutations, present in 5% to 10% of patients who have received a diagnosis of breast cancer,31forms the basis for clinical use of PARP inhibitors in treatment of breast and other cancers characterized by mutations/defects in the DNA-repair pathway. In 2018, 2 PARP inhibitors, olaparib (Lynparza) and talazoparib (Talzenna), were approved for deleterious or suspected deleterious germlineBRCA-mutated (gBRCAm) HER2-negative metastatic breast cancer.32,33
Olaparib
The FDA’s first approval of a treatment for patients with gBRCAm HER2-negative metastatic breast cancer was based on data from the OlympiAD trial, which demonstrated that PARP inhibitor monotherapy provided a significant benefit over standard therapy among patients with HER2-negative gBRCAm metastatic breast cancer.31
Subsequent analyses indicated no statistically significant improvement in OS with olaparib; however, potential for meaningful OS benefit among patients who had not received chemotherapy for metastatic disease was noted, with no evidence of cumulative toxicity during extended exposure.34
At this year’s ASCO meeting, data from the phase II GeparOLA trial of olaparib plus paclitaxel followed by epirubicin/cyclophosphamide as neoadjuvant treatment of HER2-negative early breast cancer with homologous recombination deficiency (HRD) indicated that olaparib may have expanded clinical utility in certain patient subgroups (FIGURE).35Stratified sub-group analysis showed higher pathologic complete response rates with the olaparib regimen in the cohorts of patients <40 years and in HR-positive patients.35
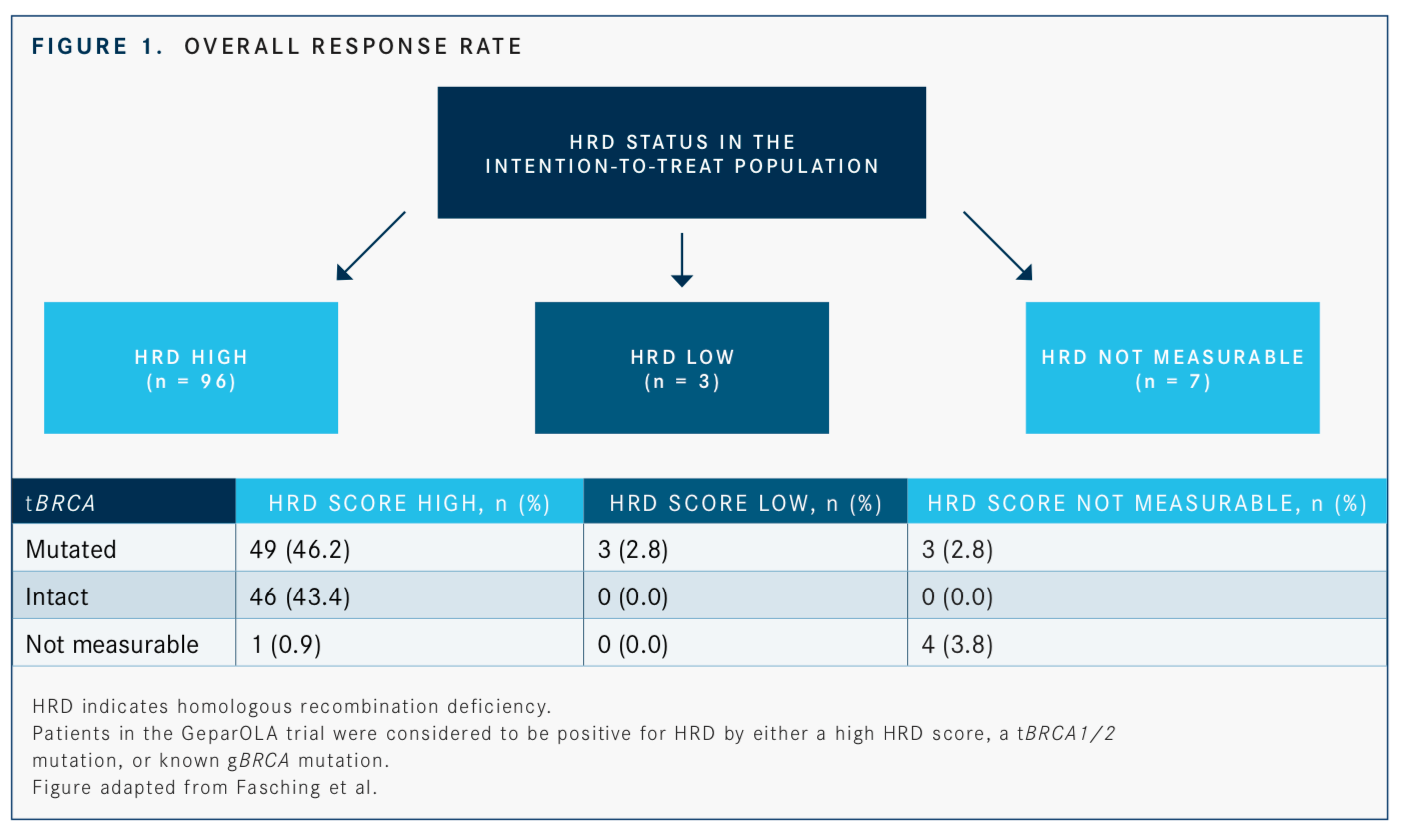
“In the subgroup analysis, which are hypothesis generating, we could see a pronounced effect in favor of olaparib in HR-positive tumors, in patients who were younger than 40 years, and in patients who had an HRD score [that] was high with a mutation in theBRCA1orBRCA2gene,” said Peter A Fasching, MD, lead author. “The results are good enough to pursue this treatment option further in the neoadjuvant setting.”
Talazoparib
Talazoparib has been shown to exhibit a high degree of potency and oral bioavailability, com- pared with other clinical PARP inhibitors.31Its FDA approval was based on data from the pivotal EMBRACA study, in which 431 patients withgBRCAmHER2-negative locally advanced or metastatic breast cancer were randomized 2:1 to receive talazoparib or physician’s choice of chemotherapy. The median PFS was significantly longer in the talazoparib group than in the standard-therapy group, at 8.6 months and 5.6 months, respectively (hazard ratio, 0.54; 95% CI, 0.41-0.71;P<.001).33
Although the PARP inhibitor regulatory approval is currently restricted to patients withBRCAmutations, studies have indicated that other mutations may also render tumors sensitive to PARP inhibitors, pointing to their potential beyond the context ofBRCAmutations.36
Data from a proof-of-concept phase II study, which evaluated talazoparib monotherapy in patients with advanced HER2-negative breast cancer or other solid tumors with a germline or somatic alteration in homologous recombination pathway genes not includingBRCA1/2, were recently reported at the 2019 ASCO Annual Meeting. In this study, patients with measurable disease and at least 1 prior therapy for advanced HER2-negative tumors with a mutation in 1 of the DNA-repair genes were treated with talazoparib until disease progression.37
“We know based on basic science, as well as clinical trials in other disease settings, that there are a number of mutations outside canonicalBRCA1/2that could potentially lead to HRD and therefore, through similar mechanisms, sensitize [the tumor] to PARP inhibitor therapy,” Gruber said. “This trial was designed to survey for those mutations, identify them in metastatic biopsies, and select patients for treatment based on this biomarker. The relevance is that you have a certain set of patients with mutations aside fromBRCA1/2who could be good candidates for PARP inhibitor therapy.” Of 13 patients with breast cancer, which included 1 patient with triple-negative disease, 4 had a partial response (PR) to therapy and 3 had stable disease (SD) ≥6 months, for a clinical benefit rate of 54%. Of those, 4 patients (3 PRs and 1 SD ≥6 months) had mutations ingPALB2, suggesting that this and other HRD alterations may be useful biomarkers for predicting talazoparib response.37
“There does seem to be a signal for gPALB2 mutations, [and] there is possibly also a signal forCHEK2mutations,” Gruber said. “All of these signals need to be identified and studied more carefully in further trials.”
The antitumor effects of PARP inhibition on cancers with defects in other DNA-repair pathway genes, and even in those without deficits in DNA repair, suggest a possible broader role for PARP inhibitors.36The problem of identifying and incorporating predictive biomarkers that may distinguish patients likely to respond to PARP inhibitors and other targeted monotherapies or combinations is currently being unraveled.
Utility of Genomic Biomarkers in ER-Positive Tumors
Given the significant challenge of primary or secondary resistance to endocrine therapy, biomarkers predictive of early progression on endocrine therapy and of response to new and emerging targeted therapies are of great interest.
A recent report described analysis of somatic mutations, assessed with a 17-gene panel, in baseline plasma samples from the phase III PALOMA-3 trial, which compared fulvestrant plus either palbociclib or placebo. Analysis of associations between mutations and copy number aberrations to clinical characteristics and PFS indicatedTP53mutation,FGFR1amplification, and tumor purity in plasma as markers for early progression.38
Ben O’Leary, MBBS, who presented the data at ASCO’s 2019 meeting, said, “These results in the future could perhaps inform clinical trials in advanced ER-positive breast cancer to identify a high-risk group of patients that may require escalation of therapy.”
References
- Presti D, Quaquarini E. The PI3K/AKT/mTOR and CDK4/6 pathways in endocrine resistant HR+/HER2- metastatic breast cancer: biological mechanisms and new treatments.Cancers(Basel). 2019;11(9):pii:E1242. doi: 10.3390/cancers11091242.
- Howlader N, Altekruse SF, Li CI, et al. US incidence of breast cancer subtypes defined by joint hormone receptor and HER2 status.J Natl Cancer Inst. 2014;106(5). doi: 10.1093/jnci/dju055.
- El Sayed R, El Jamal L, El Iskandarani S, Kort J, Abdel Salam M, Assi H. Endocrine and targeted therapy for hormone-receptor-positive, HER2-negative advanced breast cancer: insights to sequencing treatment and overcoming resistance based on clinical trials.Front Oncol. 2019;9:510. doi: 10.3389/fonc.2019.00510.
- Novartis Kisqali significantly prolongs life in women with HR+/HER2-advanced breast cancer now in two distinct phase III trials [news release]. Basel, Switzerland: Novartis; July 31, 2019. bit.ly/2GEKYvQ. Accessed September 4, 2019.
- Im S-A, Lu Y-S, Bardia A, et al. Overall survival with ribociclib plus endocrine therapy in breast cancer. N Engl J Med. 2019;381(4):307-316. doi: 10.1056/NEJMoa1903765.
- Martín M, Johnston S, Huober J, et al. MONARCH 3: Updated time to chemotherapy and disease progression following abemaciclib plus aromatase inhibitor (AI) in HR+, HER2-advanced breast cancer.Annals of Oncology(2019) 30 (suppl_5): v104-v142. 10.1093/annonc/mdz242.
- FDA approves alpelisib for metastatic breast cancer. FDA website. bit.ly/30Lzsak. Updated May 28, 2019. Accessed September 4, 2019.
- Pfizer receives US FDA accelerated approval of Ibrance (palbociclib) [news release]. New York, NY: Pfizer; February 3, 2015. bit.ly/2zxgshS. Accessed September 10, 2019.
- Hortobagyi GN, Stemmer SM, Burris HA, et al. Ribociclib as first-line therapy for HR-positive, advanced breast cancer.N Engl J Med. 2016;375(18):1738-1748. doi: 10.1056/NEJMoa1609709.
- Ribociclib (Kisqali). FDA website. bit.ly/31QF83v. Updated March 14, 2017. Accessed September 4, 2019.
- Goetz MP, Toi M, Campone M, et al. MONARCH 3: abemaciclib as initial therapy for advanced breast cancer.J Clin Oncol. 2017;35(32):3638-3646. doi: 10.1200/JCO.2017.75.6155.
- FDA approves abemaciclib as initial therapy for HR-positive, HER2-negative metastatic breast cancer. FDA website. bit.ly/2kfBOxW. Published February 26, 2018. Accessed September 5, 2019.
- Slamon DJ. Overall survival (OS) results of the phase III MONALEESA-3 trial of postmenopausal patients (pts) with hormone receptor-positive (HR+), human epidermal growth factor 2-negative (HER2-) advanced breast cancer (ABC) treated with fulvestrant (FUL) + ribociclib (rib). Presented at: ESMO Congress 2019. September 27-October 1, 2019. Barcelona, Spain. Abstract LBA7_PR.
- Slamon DJ, Neven P, Chia S, et al. Phase III randomized study of ribociclib and fulvestrant in hormone receptor-positive, human epidermal growth factor receptor 2-negative advanced breast cancer: MONALEESA-3.J Clin Oncol. 2018;36(24):2465-2472. doi: 10.1200/JCO.2018.78.9909.
- Smith A. NICE U-turn for Kisqali in breast cancer.PharmaTimes.July 17, 2019. bit.ly/2kaAV9P. Accessed September 5, 2019.
- Marra A, Curigliano G. Are all cyclin-dependent kinases 4/6 inhibitors created equal?NPJ Breast cancer. 2019;5:27. doi: 10.1038/s41523-019-0121-y.
- Sahebjam S, Le Rhun E, Kulanthaivel P, et al. Assessment of concentrations of abemaciclib and its major active metabolites in plasma, CSF, and brain tumor tissue in patients with brain metastases secondary to hormone receptor positive (HR+) breast cancer.J Clin Oncol. 2016;34(suppl 15; abstr 526). doi: 10.1200/JCO.2016.34.15_suppl.526.
- Anders CK, Le Rhun E, Bachelot TD, et al. A phase II study of abemaciclib in patients (pts) with brain metastases (BM) secondary to HR+, HER2- metastatic breast cancer (Mbreast cancer).J Clin Oncol. 2019;37(suppl 15; abstr 1017). doi: 10.1200/JCO.2019.37.15_suppl.1017.
- Sledge G. Monarch 2: overall survival of abemaciclib plus fulvestrant in patients with HR+, HER2-advanced breast cancer. Presented at: ESMO Congress 2019. September 27-October 1, 2019. Barcelona, Spain. Abstract LBA6_PR.
- FDA approves abemaciclib for HR-positive, HER2-negative breast cancer. FDA website. bit.ly/2kaoDyd. Published September 28, 2017. Accessed September 5, 2019.
- Liu P, Cheng H, Roberts TM, Zhao JJ. Targeting the phosphoinositide 3-kinase pathway in cancer.Nat Rev Drug Discov. 2009;8(8):627-644. doi: 10.1038/nrd2926.
- Dey N, De P, Leyland-Jones B. PI3K-AKT-mTOR inhibitors in breast cancers: from tumor cell signaling to clinical trials.Pharmacol Ther. 2017;175:91-106. doi: 10.1016/j.pharmthera.2017.02.037.
- Ballinger TJ, Meier JB, Jansen VM. Current landscape of targeted therapies for hormonereceptor positive, HER2 negative metastatic breast cancer.Front Oncol. 2018;8:308. doi: 10.3389/fonc.2018.00308.
- Cuorvo LV, Verderio P, Ciniselli CM, et al. PI3KCA mutation status is of limited prognostic relevance in ER-positive breast cancer patients treated with hormone therapy.Virchows Arch. 2014;464(1):85-93. doi: 10.1007/s00428-013-1500-7.
- Juric D, Krop I, Ramanathan RK, et al. Phase I dose escalation study of taselisib (GDC-0032), an oral PI3K inhibitor, in patients with advanced solid tumors.Cancer Discov. 2017;7(7):704-715. doi: 10.1158/2159-8290.CD-16-1080.
- Carroll J. Roche dumps its PhIII PI3K effort on taselisib after researchers track poor survival edge, harsh side effects for breast cancer. Endpoints News website. bit.ly/2mfZi6N. Published June 3, 2018. Accessed September 11, 2019.
- 2018 ASCO: phase III SANDPIPER trial evaluates taselisib plus fulvestrant in advanced breast cancer. The ASCO Post website. bit.ly/2m526Dn. Updated June 6, 2018. Accessed September 10, 2019.
- Nur Husna SM, Tan H-TT, Mohamud R, Dyhl-Polk A, Wong KK. Inhibitors targeting CDK4/6, PARP and PI3K in breast cancer: a review.Ther Adv Med Oncol. 2018;10:1758835918808509. doi: 10.1177/1758835918808509.
- André F, Ciruelos E, Rubovszky G, et al; SOLAR-1 Study Group. Alpelisib forPIK3CA-Mutated, Hormone Receptor-Positive Advanced Breast cancer.N Engl J Med. 2019;380(20):1929-1940. doi: 10.1056/NEJMoa1813904.
- Savard M-F, Khan O, Hunt KK, Verma S. Redrawing the lines: the next generation of treatment in metastatic breast cancer.Am Soc Clin Oncol Educ Book. 2019;39:e8-e21. doi: 10.1200/EDBK_237419.
- Exman P, Barroso-Sousa R, Tolaney SM. Evidence to date: talazoparib in the treatment of breast cancer.Onco Targets Ther. 2019;12:5177-5187. doi: 10.2147/OTT.S184971.
- FDA approves olaparib for germline BRCA-mutated metastatic breast cancer. FDA website. bit.ly/2kFaAAU. Published January 21, 2018. Accessed September 11, 2019.
- FDA approves talazoparib for gBRCAm HER2-negative locally advanced or metastatic breast cancer. FDA website. bit.ly/2kEhzKp. Updated December 14, 2018. Accessed September 11, 2019.
- Robson ME, Tung N, Conte P, et al. OlympiAD final overall survival and tolerability results: olaparib versus chemotherapy treatment of physician’s choice in patients with a germline BRCA mutation and HER2-negative metastatic breast cancer.Ann Oncol. 2019;30(4):558-566. doi: 10.1093/annonc/mdz012.
- Fasching PA, Jackisch C, Rhiem K, et al. GeparOLA: a randomized phase II trial to assess the efficacy of paclitaxel and olaparib in comparison to paclitaxel/carboplatin followed by epirubicin/cyclophosphamide as neoadjuvant chemotherapy in patients (pts) with HER2-negative early breast cancer (breast cancer) and homologous recombination deficiency (HRD).J Clin Oncol. 37(suppl 15; abstr 506). doi: 10.1200/JCO.2019.37.15_suppl.506.
- Shao N, Shi Y, Yu L, et al. Prospect for application of PARP inhibitor in patients with HER2 negative breast cancer.Int J Biol Sci. 2019;15(5):962-972. doi: 10.7150/ijbs.30721.
- Gruber JJ, Afghahi A, Hatton A, et al. Talazoparib beyond BRCA: a phase II trial of talazoparib monotherapy inBRCA1andBRCA2wild-type patients with advanced HER2-negative breast cancer or other solid tumors with a mutation in homologous recombination (HR) pathway genes.J Clin Oncol. 37(suppl 15; abstr 3006). doi: 10.1200/JCO.2019.37.15_suppl.3006
- O’Leary B, Cutts R, Huang X, et al. Genomic markers of early progression on fulvestrant with or without palbociclib for ER+ advanced breast cancer in the PALOMA-3 trial.J Clin Oncol. 37(suppl 15; abstr 1010). doi: 10.1200/JCO.2019.37.15_suppl.1010.
Breast Cancer Leans into the Decade of Antibody-Drug Conjugates, Experts Discuss
September 25th 2020In season 1, episode 3 of Targeted Talks, the importance of precision medicine in breast cancer, and how that vitally differs in community oncology compared with academic settings, is the topic of discussion.
Listen
Overcoming Barriers in Cancer Clinical Trials: A Path Forward for Better Patient Care
April 29th 2024Clinical trials play a pivotal role in developing effective therapies, yet their integration is challenged by issues such as insufficient reimbursement structures, misaligned incentives, physician burnout, and a complex regulatory environment.
Read More