April Roundtable Roundup: B-cell Lymphoma
In separate, live virtual events, Kami J. Maddocks, MD, and Sairah Ahmed, MD, discussed the use of loncastuximab tesirine for a patient with later-line diffuse large B-cell lymphoma.
CASE SUMMARY
- A 73-year-old woman presented with fever, headaches, and a 7-lb unintentional weight loss .
- Family history: married with 2 grown children who live in other states; primary caretaker for her mother who has advanced dementia; no family history of cancer
- Medical history: hyperlipidemia well controlled with simvastatin
- Physical exam: palpable bilateral cervical lymphadenopathy
- Laboratory results: lactate dehydrogenase, 265 U/L (upper limit, 280 U/L); hemoglobin, 10.8 g/dL; bilirubin, 1.3 mg/dL (upper limit, 1.2 mg/dL); creatinine, 1.7 mg/dL (upper limit, 1.2 mg/dL); all others within normal limit
- Hepatitis B, C, and HIV negative
- Lymph node biopsy; immunohistochemistry panel: CD10+, CD20+, which confirmed diffuse large B-cell lymphoma (DLBCL)
- Fluorescence in situ hybridization: negative for rearrangements of BCL6, BCL2, and C-MYC
- Whole body PET/CT scan showed diffuse adenopathy, largest node 3.9 cm
- MRI of the brain showed no evidence of lesions
- ECOG performance status: 1
- Stage III disease; International Prognostic Index score 2; low-intermediate risk
- The patient received 6 cycles of R-CHOP (rituximab [Rituxan], cyclophosphamide, doxorubicin hydrochloride, and vincristine sulfate [Oncovin], and prednisone), which she tolerated well.
- She had a complete response (CR) at the end of treatment, but 14 months later she presented with diffuse lymphadenopathy, confirmed by PET/CT scan .
- Biopsy showed relapse of DLBCL
- The patient received 6 cycles of polatuzumab vedotin (Polivy), bendamustine, and rituximab (Pola-BR).
- She achieved response, then progressed 8 month s later.
- The patient is not able to travel because she is the primary caretaker for her mother, who has dementia.
- Current treatment: loncastuximab tesirine-lpyl (Zynlonta)
EVENT REGION Kentucky, Michigan, Illinois, Indiana, Ohio, and Wisconsin
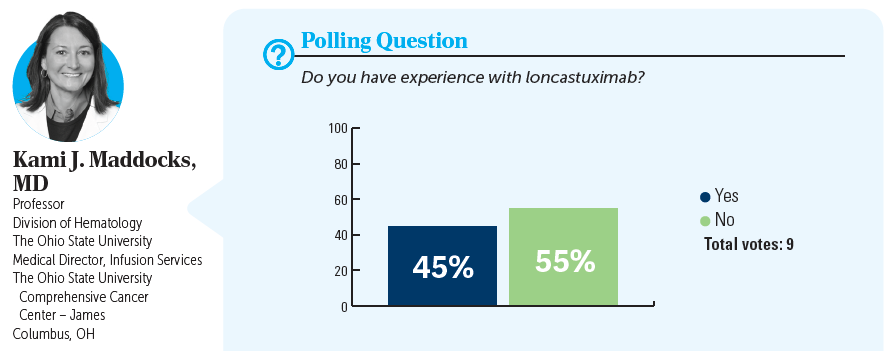
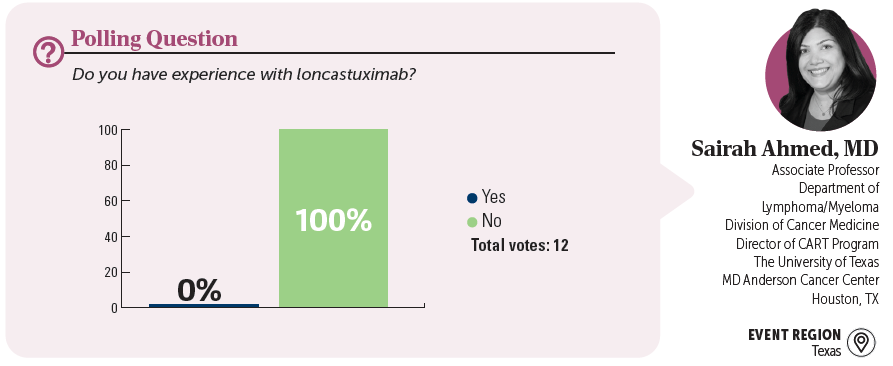
KAMI J. MADDOCKS, MD: Do you agree with this treatment choice? Who has heard of or used loncastuximab?
MOHAMAD K. KHASAWNEH, MD: This is a scenario that I would seriously consider loncastuximab for because the patient seems to know that treatment is palliative at this time. She’s not looking into any intensive treatment with the hope of cure. She has a difficult social situation of taking care of her mom. I have used single-agent loncastuximab [before], and it’s definitely a great option at this time because of its mechanism of action. It does have its own adverse event [AE] profile, like fluid overload, fatigue, and some myelosuppression, [although] not as high as we see with chemotherapy or tafasitamab-cxix [Monjuvi] and lenalidomide [Revlimid].
NEERAJ MAHAJAN, MD: I have used loncastuximab and it turned out to be the most practical and logistically feasible single agent, given every 3 weeks, 30 to 45 minutes infusion, with no premedications, so you don’t have to combine it with BR and you don’t have to get [patients] approved for lenalidomide. AEs are there, but compared with other options, I think they are still manageable. At least for a lot of these patients who have these issues with travel and comorbidities, that is a reasonable choice; at least in the community setting and from the scheduling and doing the infusions for those patients, it is the most convenient of [the treatment options].
MARK H. KNAPP, MD: I’ve also used it. It seems very convenient for the patient every 3 weeks—a short infusion. The patient I had tolerated it, is on it currently, and tolerated it much better than their second-line therapy.

Leon-Ferre Explores Targeting of PIK3CA Alterations in ER+ Breast Cancer
July 24th 2024During a live Community Case Forum event in partnership with the Minnesota Society of Clinical Oncology, Roberto A. Leon-Ferre, MD, discussed drugs targeting PIK3CA alterations in patients with ER+ metastatic breast cancer.
Read More
George Explores Impact of Risk Status With Cabozantinib/Nivolumab in Advanced RCC
July 19th 2024During a Case-Based Roundtable® event, Daniel George, MD, discussed the results of the CheckMate 9ER trial across favorable, intermediate, and poor risk groups in patients with advanced renal cell carcinoma.
Read More
Depth of Response With Quadruplet Regimens Considered in Newly Diagnosed Multiple Myeloma
July 18th 2024During a Case-Based Roundtable® event, Timothy Schmidt, MD, and participants discussed treatment selection for a 54-year-old patient with transplant eligible R-ISS stage 2/R2-ISS stage 3 IgG-κ myeloma.
Read More
Rossetti Reviews Myelofibrosis Risk Stratification and Outcome Data for Pacritinib
July 17th 2024During a Case-Based Roundtable® event, James M. Rossetti, DO, discussed the role of risk scoring and stratification tools and treatment for a patient with declining hemoglobin and platelet counts due to primary myelofibrosis.
Read More