Rizzieri Looks at Long-Term Efficacy of Combination Therapy in DLBCL
At a live virtual event, David Rizzieri, MD, CPE, provided commentary on the landscape of treatment for patients with relapsed/refractory diffuse large B-cell lymphoma (DLBCL).
David Rizzieri, MD, CPE
Senior Vice President
System Physician Executive
Hematology and Oncology
Novant Health Agnes B. and Edward I. Weisiger Cancer Institute

At a live virtual event, David Rizzieri, MD, CPE, provided commentary on the landscape of treatment for patients with relapsed/ refractory diffuse large B-cell lymphoma (DLBCL). He highlighted the importance of understanding all the data for the options physicians have, knowing that something may show great responses early on but not have long-term durability. This is why the data from the multicenter, open-label, single arm, phase 2 L-MIND (NCT02399085) study are important to note when looking to treat patients for whom 1 or more lines of prior therapy failed. These data showed long-term survival after 5 years of treatment and a toxicity profile that was manageable after handling tougher upfront adverse events.
LOOKING AT THE GUIDELINES
RIZZIERI: I’m old enough to remember...in the early 90s...that we all thought the third-generation regimens we used at the time were going to be better than cyclophosphamide, doxorubicin hydrochloride, and vincristine sulfate [Oncovin; CHOP].1 It was [therapies like] the proMACE-cytaBOM [cyclophosphamide, doxorubicin, etoposide, cytarabine, bleomycin, vincristine, methotrexate, prednisone, leucovorin, and co-trimoxazole]...that had improved progression-free survival [PFS], and not a single one improved overall survival [OS], but they were all more toxic than CHOP.1
When we look at polatuzumab vedotin [Polivy] plus bendamustine [Bendeka] and rituximab [Rituxan; Pola-BR] it also has a better PFS, but we have no data on improved OS and the same could be said for dose-adjusted rituximab-etoposide phosphate, vincristine sulfate, cyclophosphamide, doxorubicin hydrochloride, and prednisone [R-EPOCH].2,3 [Pola-BR is] going to give you a better complete response [CR] rate and the data would suggest PFS improvements in most subgroups, but no change in survival in phase 3 [studies]. And I don’t think it’s wrong to do Pola-BR, but it’s not a given, and I’m not moved off the blocks to switch everybody to it but some people are.... It’s certainly still reasonable and one of the reasons it’s still an option [under the National Comprehensive Cancer Network (NCCN) guidelines].
NCCN recommendations for [patients with DLBCL] at stage I early disease would be R-CHOP.4 At stage II with extensive mesenteric disease or stage III-IV—so your more advanced-stage patients—the preferred regimens are R-CHOP, R-CHP, and dose-adjusted R-EPOCH, which are all very reasonable. We also have several other regimens for those patients with cardiac dysfunction [as well as DLBCL], and we’re all aware of these regimens. I would say we’re aware of the limited data that suggest that the infusion of anthracyclines may be less cardiotoxic [for patients],5 although we haven’t really proved that in any good large studies.
LONG-TERM DATA SHOWING EFFECTIVENESS OF COMBINATION THERAPY
The original approval of the tafasitamab [Monjuvi] plus lenalidomide [Revlimid] combination from the FDA [was due to the] 1-year data from the L-MIND study,6 but now the 5-year data...[have shown] that in those patients with 2 or more prior lines of therapy, the overall response rates [ORRs] were 47.5% [95% CI, 31.5%-63.9%] with a 30% CR rate.7
If they had just 1 prior line of therapy that failed them, the CR rate is about 52.5% with an ORR of 67.5% [95% CI, 45.9%-79.4%].7 If we think about the patient in this [case] who progressed at 8 months and wanted to get therapy at home, and declined chimeric antigen receptor [CAR] T-cell therapy, compared with other chemotherapy options [these data are] encouraging to me when we look at the 5-year results, because there’s a meaningful chance of a true CR [ for the patient]. Now that’s nice but what we’re really looking for is durability, and the median duration of response [DOR] at the 5-year follow-up...is not met [95% CI, 33.8-not reached (NR)].7
[To answer the] question of are we curing some of these people—only if they fall into the subgroup of a full remission, and we have 5-year data that have over 50% [of patients] still in remission....7 We have a lot of patients [receiving] CAR T-cell therapy [who are] still progressing but there’s a thought that some of them might be cured, and maybe with this immunotherapy there’s that similar idea but I don’t know. But certainly, if you have a patient [receiving] tafasitamab/lenalidomide and they get a remission [with the therapy], then you can tell the patient if they’re in that group then there is more than a 50% chance they’re going to maintain that remission beyond 5 years, and that’s awfully encouraging from my perspective.
Now, CRs are still important, but if we [can get the patient to] have a long survival with stable disease for this patient population, that’s ideal, and that’s the kind of data we have with a 5-year follow-up here. Again, [in patients for whom] 1 prior line of therapy [ failed] vs 2 or more,… there was certainly a difference [in their follow-up and OS results] at median follow-up of 57.6 months [95% CI, 26.5-60.7] vs 33.9 months [95% CI, 10.9-46.8], respectively.7 In the final 5-year data, the median OS was NR [95% CI, 24.6-NR] if the patient had 1 prior line of therapy, and if they had 2 or more prior lines then the median OS was 15.5 months [95% CI, 8.6-45.5].6 Again, the point of this would suggest PFS improvements in most subgroups, but no change in survival in phase 3 [studies]. And I don’t think it’s wrong to do Pola-BR, but it’s not a given, and I’m not moved off the blocks to switch everybody to it but some people are.... It’s certainly still reasonable and one of the reasons it’s still an option [under the National Comprehensive Cancer Network (NCCN) guidelines].
NCCN recommendations for [patients with DLBCL] at stage I early disease would be R-CHOP.4 At stage II with extensive mesenteric disease or stage III-IV—so your more advanced-stage patients—the preferred regimens are R-CHOP, R-CHP, and dose-adjusted R-EPOCH, which are all very reasonable. We also have several other regimens for those patients with cardiac dysfunction [as well as DLBCL], and we’re all aware of these regimens. I would say we’re aware of the limited data that suggest that the infusion of anthracyclines may be less cardiotoxic [for patients],5 although we haven’t really proved that in any good large studies.
LONG-TERM DATA SHOWING EFFECTIVENESS OF COMBINATION THERAPY
The original approval of the tafasitamab [Monjuvi] plus lenalidomide [Revlimid] combination from the FDA [was due to the] 1-year data from the L-MIND study,6 but now the 5-year data...[have shown] that in those patients with 2 or more prior lines of therapy, the overall response rates [ORRs] were 47.5% [95% CI, 31.5%-63.9%] with a 30% CR rate.7
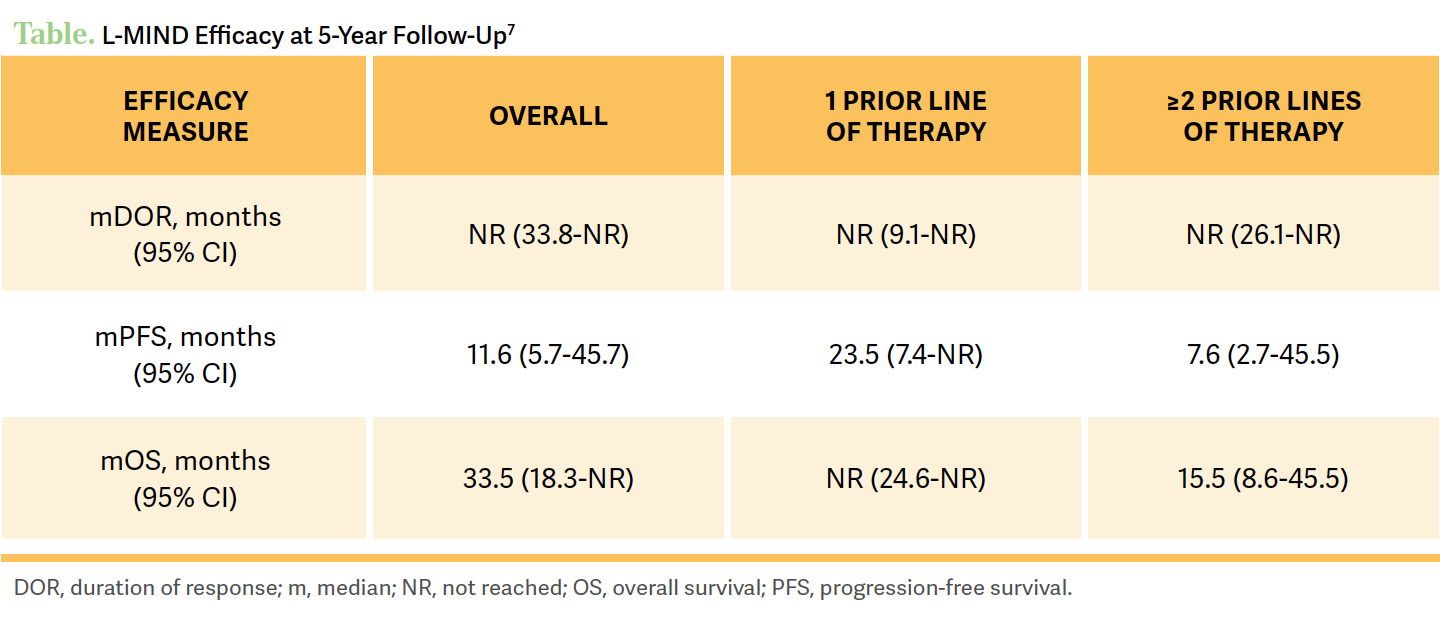
If they had just 1 prior line of therapy that failed them, the CR rate is about 52.5% with an ORR of 67.5% [95% CI, 45.9%-79.4%].7 If we think about the patient in this [case] who progressed at 8 months and wanted to get therapy at home, and declined chimeric antigen receptor [CAR] T-cell therapy, compared with other chemotherapy options [these data are] encouraging to me when we look at the 5-year results, because there’s a meaningful chance of a true CR [for the patient]. Now that’s nice but what we’re really looking for is durability, and the median duration of response [DOR] at the 5-year follow-up...is not met [95% CI, 33.8-not reached (NR)].7
[To answer the] question of are we curing some of these people—only if they fall into the subgroup of a full remission, and we have 5-year data that have over 50% [of patients] still in remission....7 We have a lot of patients [receiving] CAR T-cell therapy [who are] still progressing but there’s a thought that some of them might be cured, and maybe with this immunotherapy there’s that similar idea but I don’t know. But certainly, if you have a patient [receiving] tafasitamab/lenalidomide and they get a remission [with the therapy], then you can tell the patient if they’re in that group then there is more than a 50% chance they’re going to maintain that remission beyond 5 years, and that’s awfully encouraging from my perspective.
Now, CRs are still important, but if we [can get the patient to] have a long survival with stable disease for this patient population, that’s ideal, and that’s the kind of data we have with a 5-year follow-up here. Again, [in patients for whom] 1 prior line of therapy [ failed] vs 2 or more,… there was certainly a difference [in their follow-up and OS results] at median follow-up of 57.6 months [95% CI, 26.5-60.7] vs 33.9 months [95% CI, 10.9-46.8], respectively.7 In the final 5-year data, the median OS was NR [95% CI, 24.6-NR] if the patient had 1 prior line of therapy, and if they had 2 or more prior lines then the median OS was 15.5 months [95% CI, 8.6-45.5].6 Again, the point of this therapy was to [have the patient] stay on therapy until it failed them, but a lot of people stopped treatment for a variety of reasons. But when they did stop many still maintained a remission that was quite durable [through the 5-year mark], but it wasn’t everybody who maintained it.7
ADDRESSING TOXICITIES
[Now the tafasitamab/lenalidomide had] nice efficacy results at 5 years, but is it toxic? Are these people going to be able to stay home, receive outpatient care, and have a good quality of life? Looking at the treatment-emergent adverse events [TEAEs] across all treatments, including TEAEs from the combined therapy or lenalidomide, there were 851 any-grade nonhematologic TEAEs and 345 hematologic TEAEs [over the entirety of 5 years]. Looking at the tafasitamab monotherapy given for up to 2 years, when you drop the lenalidomide the nonhematologic toxicities go down quite significantly to 383 events, which is probably not surprising, and beyond 2 years even lower at 347 events.
With the hematologic toxicities, you should primarily expect neutropenia... and with the combined therapy, that was the biggest TEAE for us to manage [with 49.4% of patients experiencing any-grade neutropenia, 48.1% of which was grade 3 or greater].7 For the monotherapy— these were the patients who were able to stay on therapy with some dosing of lenalidomide—the majority [of patients] went down to under 20 events and stayed at their same dose level.
Once [the lenalidomide is] dropped you still get some neutropenia...but very few high-grade cases, and occasionally beyond 2 years of tolerating treatment this well, again [it] is uncommon. The most common [any-grade nonhematologic TEAEs] during the combination phase were diarrhea [37.0%], asthenia [25.9%], and peripheral edema [24.7%].7 Once the lenalidomide was dropped, and then beyond 2 years of monotherapy, there were very few nonhematologic toxicities encountered.
Now,…we forget that lenalidomide still has a whole package insert for us to worry about. So I think concerns regarding renal function issues in these patients are appropriate concerns, and many of the patients do have to have a dose reduction of lenalidomide. I do think in those first 3 treatment cycles it’s every week they’re coming in and they have to get some therapy if they choose therapy, but still having logistics and support is important [in case that changes]. Having them get back and forth and having that support can be a little bit difficult.
REFERENCES
1. Rojek AE, Smith SM. Evolution of therapy for limited stage diffuse large B-cell lymphoma. Blood Cancer J. 2022;12(2):33. doi:10.1038/s41408-021-00596-z
2. Sehn LH, Hertzberg M, Opat S, et al. Polatuzumab vedotin plus bendamustine and rituximab in relapsed/refractory DLBCL: survival update and new extension cohort data. Blood Adv. 2022;6(2):533-543. doi:10.1182/bloodadvances.2021005794
3. Major A, Smith SM. DA-R-EPOCH vs R-CHOP in DLBCL: how do we choose? Clin Adv Hematol Oncol. 2021;19(11):698-709.
4. NCCN. Clinical Practice Guidelines in Oncology. B-cell lymphomas, version 1.2024. Accessed January 25, 2024. https://bit.ly/3TEXEqA
5. Teixeira da Silva F, Morais Passos R, Esteves A, Carvalho J, Ferreira M. Anthracycline cardiotoxicity in a patient with diffuse large B-cell lymphoma: a case report. Cureus. 2020;12(10):e11038. doi:10.7759/ cureus.11038
6. FDA grants accelerated approval to tafasitamab-cxix for diffuse large B-cell lymphoma. Updated August 3, 2020. Accessed January 25, 2024. http://tinyurl. com/774sy3d3
7. Duell J, Abrisqueta P, Andre M, et al. Tafasitamab for patients with relapsed or refractory diffuse large B-cell lymphoma: final 5-year efficacy and safety in the phase II L-MIND study. Haematologica. Published online August 31, 2023. doi:10.3324/ haematol.2023.283480

Leon-Ferre Explores Targeting of PIK3CA Alterations in ER+ Breast Cancer
July 24th 2024During a live Community Case Forum event in partnership with the Minnesota Society of Clinical Oncology, Roberto A. Leon-Ferre, MD, discussed drugs targeting PIK3CA alterations in patients with ER+ metastatic breast cancer.
Read More
George Explores Impact of Risk Status With Cabozantinib/Nivolumab in Advanced RCC
July 19th 2024During a Case-Based Roundtable® event, Daniel George, MD, discussed the results of the CheckMate 9ER trial across favorable, intermediate, and poor risk groups in patients with advanced renal cell carcinoma.
Read More
Depth of Response With Quadruplet Regimens Considered in Newly Diagnosed Multiple Myeloma
July 18th 2024During a Case-Based Roundtable® event, Timothy Schmidt, MD, and participants discussed treatment selection for a 54-year-old patient with transplant eligible R-ISS stage 2/R2-ISS stage 3 IgG-κ myeloma.
Read More
Rossetti Reviews Myelofibrosis Risk Stratification and Outcome Data for Pacritinib
July 17th 2024During a Case-Based Roundtable® event, James M. Rossetti, DO, discussed the role of risk scoring and stratification tools and treatment for a patient with declining hemoglobin and platelet counts due to primary myelofibrosis.
Read More